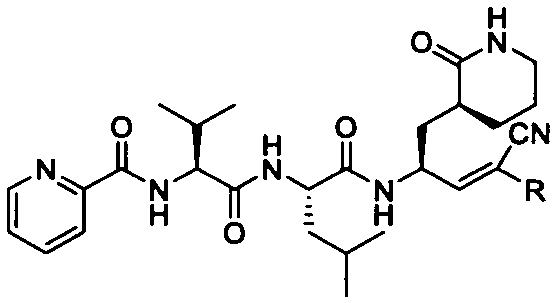
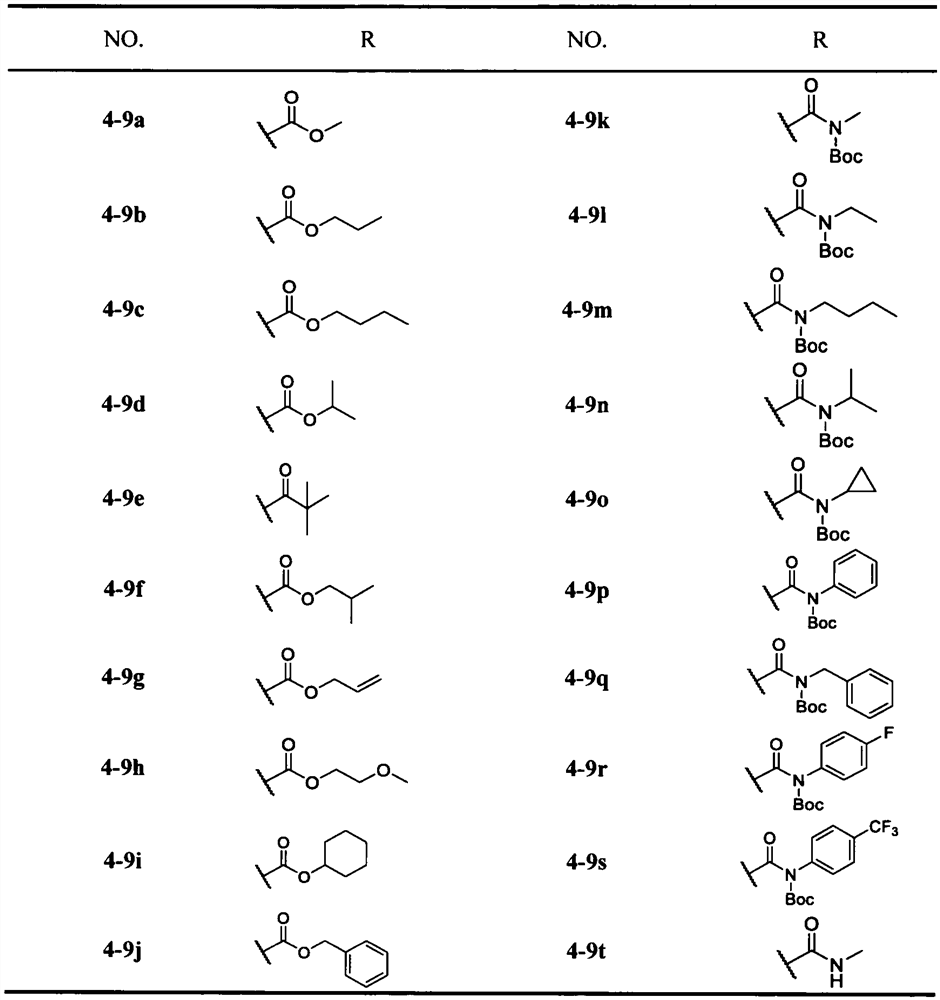
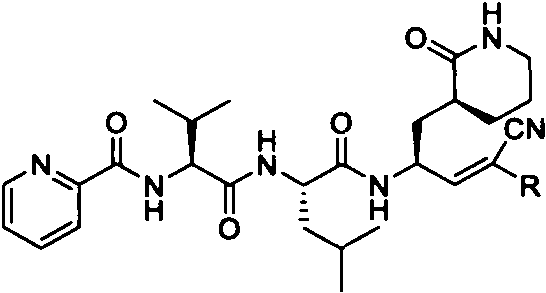
Preparation and application of Michael receptor main protease inhibitor targeting novel coronavirus
A technology of protease inhibitors and Michael receptors, which is applied in the field of preparation and application of Michael receptor-type main protease inhibitors targeting the new coronavirus, can solve the problem that virus replication and transcription cannot continue normally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0083] The present invention is illustrated in more detail by the following non-limiting examples, but the present invention is not limited to the following examples. Temperatures are provided in degrees Celsius in the following examples. Unless otherwise stated, solution percentages express a weight-to-volume relationship, and solution ratios express a volume-to-volume relationship. The structures of the compounds in the Examples were determined by one or more of the following methods: nuclear magnetic resonance spectrometer, high-resolution mass spectrometry, and thin-layer chromatography.
[0084] NMR spectrum ( 1 H NMR and 13 C NMR) were measured with a Bruker 400 spectrometer at a field strength of 400 MHz. Chemical shifts are expressed in parts per million (ppm, δ) relative to the internal standard tetramethylsilane. 1 The multiplicity of peaks in H-NMR is indicated as follows: s = singlet; d = doublet; t = triplet; m = multiplet. Coupling constants are expressed in...
Embodiment 1
[0087] Implementation Example 1: Preparation of Compound 4-1
[0088]
[0089] At 0°C, slowly add acetyl chloride (5 mL) dropwise into methanol (100 mL), stir for 5 minutes, then add glutamic acid (10 g, 67.9 mmol), continue stirring and heat to reflux, and keep the reflux temperature for 2 hours . After the reaction was complete, the solvent was distilled off under reduced pressure and recrystallized with diethyl ether. The obtained oil was dissolved in THF (150mL), TEA (28.5mL, 203.7mmol) was added dropwise at 0°C, kept stirring at 0°C for 5 minutes, and dicarbonate dicarbonate dissolved in THF (30mL) was continued to be added dropwise. Tert-butyl ester (17.8g, 81.5mmol), raised to room temperature and stirred for 2.5 hours. After the reaction, the solvent was distilled off under reduced pressure, water (200 mL) was added to the residue, and the aqueous phase was extracted with DCM (2×200 mL), the combined organic phases were dried over anhydrous sodium sulfate, and the...
Embodiment 2
[0090] Implementation Example 2: Preparation of Compound 4-2
[0091]
[0092] At -78°C, under the condition of nitrogen protection, lithium bis(trimethylsilyl)amide (78.5mL, 1.0M solution in THF, 78.5mmol) was slowly added dropwise to 4-1 (10g, 36.4mmol) without A solution of THF (200 mL) in water was stirred at this temperature for 30 minutes. Then, keeping the temperature constant, bromopropionitrile (3.4 mL) was slowly added dropwise, and the reaction mixture was stirred at -78°C for 2 hours. After the reaction was complete, glacial acetic acid (5 mL) was added to quench the reaction, and stirred to room temperature. The solvent was distilled off under reduced pressure, then water (200 mL) was added, the aqueous phase was extracted with DCM (2×200 mL), the combined organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the crude product was purified by flash chromatography (PE:EA =2:1), 4-2 was obtained as pale yellow oily liqu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com