Insulin premix formulation and product, methods of preparing same, and methods of using same
An insulin, premixed technology, applied in the manufacture and use of such insulin premixed products, can solve the problem of uncertainty about the degree to which human albumin prevents adsorption, so as to reduce medical errors , the effect of improving safety and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
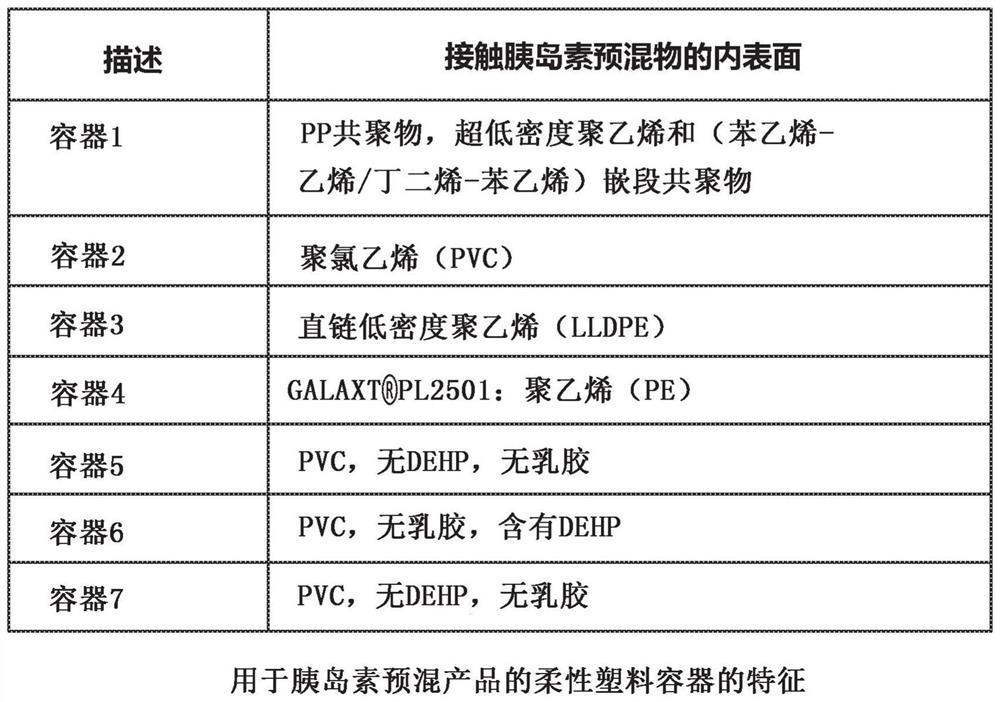
[0064] The present disclosure generally relates to a pharmaceutically acceptable insulin premix formulation comprising about 0.1-10.0 U / mL of insulin. The insulin premix formulation is an aqueous solution that can be aseptically filled into a flexible container to form a pharmaceutically acceptable insulin premix product. The insulin premix product is preferably a sterile, stable, ready-to-use aqueous solution. The insulin premix product is preferably a single-use product. The insulin premix product is preferably clear and colorless. The insulin product can be administered to an individual in need thereof for improved glycemic control therapy. The individual may be a mammal, preferably a human, including adults and children. The individual may be an individual with a metabolic disorder, including an individual in an intensive care unit (ICU) or an individual with diabetes such as type I and type II diabetes.
[0065] One aspect of the invention is a pharmaceutically accept...
Embodiment 1
[0159] A study investigated the effect of buffer and storage conditions on the stability of different insulin premix formulations. Recombinant human insulin is manufactured by microbial synthesis. The test and control articles were stored at two temperatures of about 5°C and about 25°C for up to 25.5 months (110 weeks) and at about 40°C for up to 24 weeks.
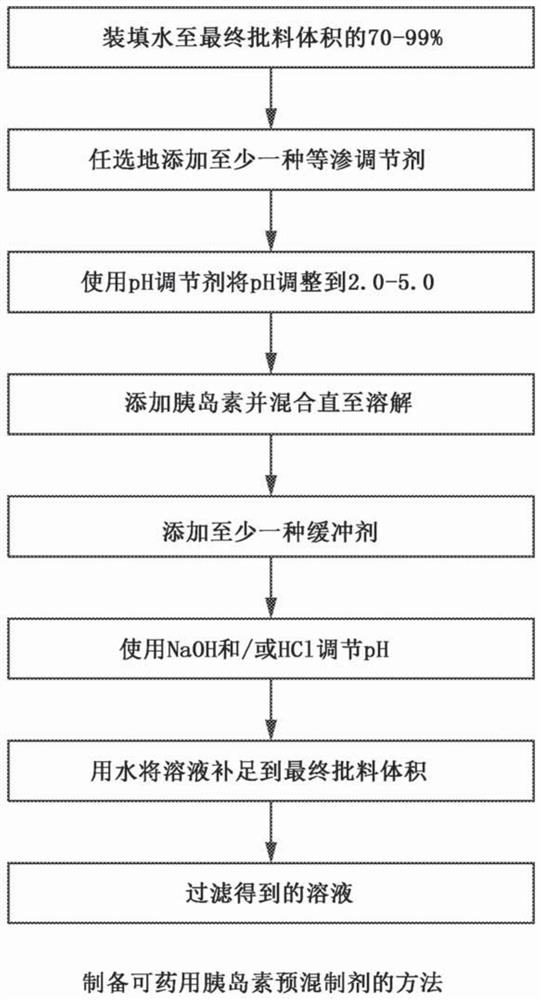
[0160] sample according to image 3 Prepared as shown in , the following mixing procedure was used for 8.0 liters of each 1.0 U / mL insulin batch: (1) fill a glass beaker with approximately 6.4 liters (80% capacity) of distilled water; (2) Add 0.3g of recombinant human insulin and it becomes turbid; (3) gradually add 0.1N HCl and adjust to a pH of about 3.0, stir slowly until the insulin is completely dissolved; (4) add 0.1N NaOH dropwise to adjust to a pH of about 7.4 ; (5) Add remaining excipients and stir until dissolved; and (6) Add distilled water to 8.0 L.
[0161] The prepared samples were aseptically filled into ...
Embodiment 2
[0177] One study investigated the effect of pH on the stability of insulin formulations at a concentration of 1.0 units / mL after nominal storage (approximately 5°C) and accelerated storage (approximately 25°C). Insulin used in this study is a polypeptide hormone structurally identical to regular human insulin and was manufactured by recombinant DNA technology using Pichia pastoris (yeast) as the production organism.
[0178] The formulation used in the design of this study was as follows: 1.0 U / mL insulin; 0.9% NaCl; 2.1 mM monobasic sodium phosphate; 2.9 mM disodium hydrogen phosphate; Samples were prepared in accordance with the instructions of the image 3 The method shown in , including the following mixing procedure: 1) fill a glass beaker to 90% of the final batch volume; 2) add NaCl and mix until dissolved to form a 0.9% saline solution by weight of the total formulation; 3 ) add 2.1 mM monobasic sodium phosphate and mix until dissolved; 4) add 1.0 U / mL of insulin and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com