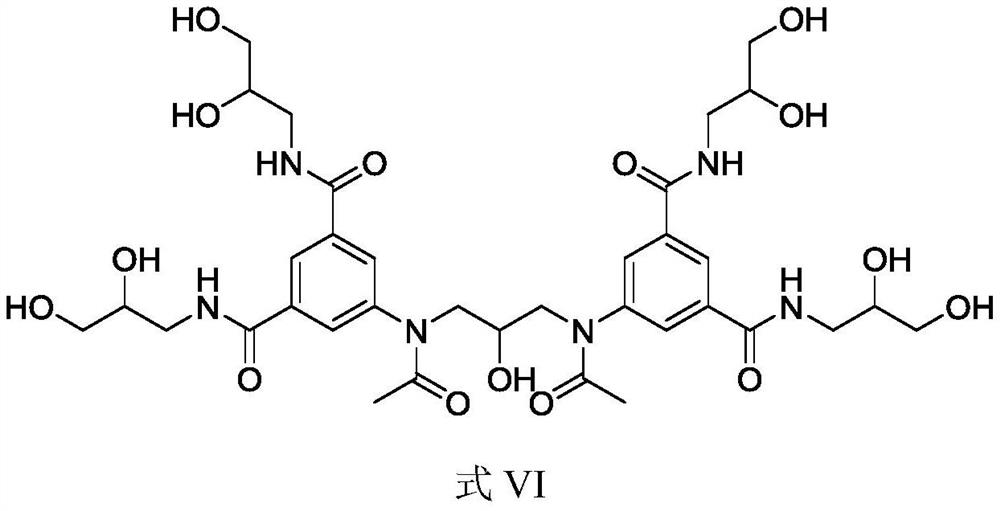
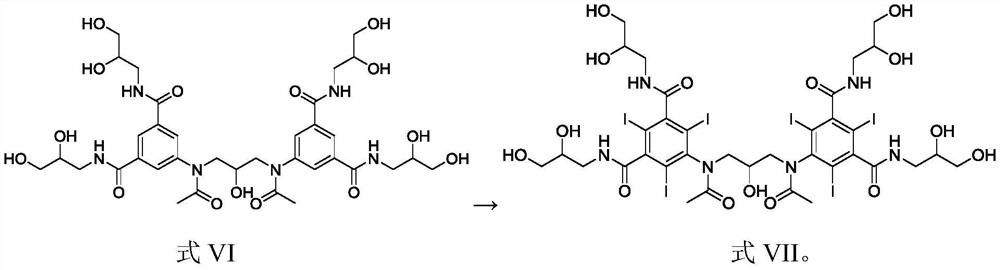
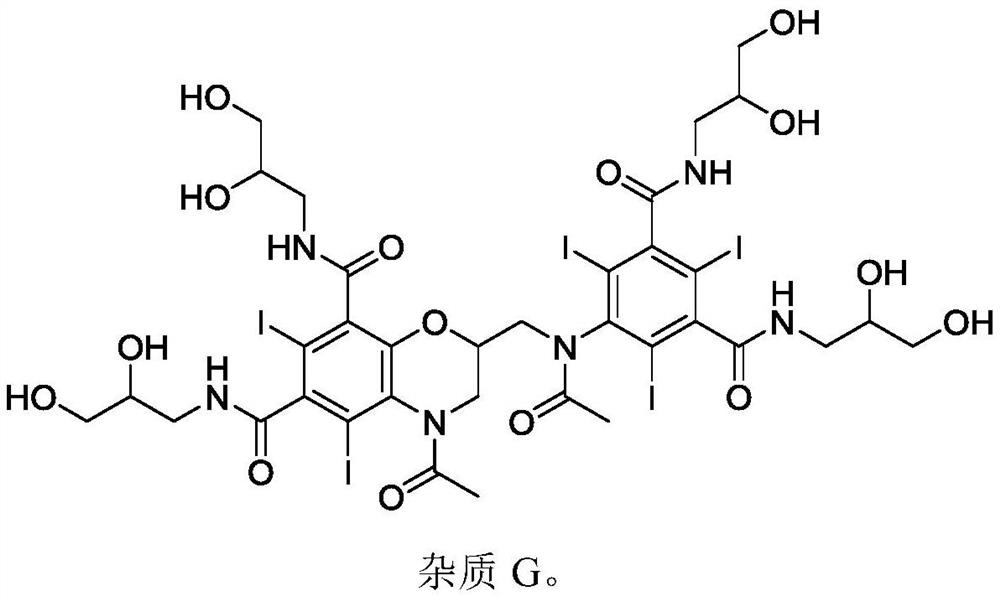
Intermediate of iodixanol and method for preparing iodixanol by using intermediate
A technology of iodixanol and intermediates, which is applied in the synthesis field of medicine and chemical industry, can solve the problems of long synthesis cycle, low purity of finished products, high equipment requirements, etc., and achieve the effect of stable product quality, low impurity content and high synthesis purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: 1) Add dimethyl 5-nitroisophthalate (850g), aminoglycerin (680g), and anhydrous methanol (680g) into a 5L three-necked glass bottle, and stir for 15 minutes. The temperature of the reaction solution was controlled at 20° C. to 30° C., and sodium methoxide methanol solution (68 g) was added slowly. The temperature of the reaction solution is controlled at 30° C. to 40° C., and the reaction is stirred for 3 to 4 hours. After the reaction is finished, the temperature of the reaction solution is lowered to 15°C to 25°C, and the temperature of the reaction solution is controlled below 30°C and glacial acetic acid is added until the pH=5.0 to 5.5, and then the reaction is completed. The 5-nitro-N,N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide (Formula II) reaction solution was obtained, which was used for the next step of production.
[0048] 2) Add the reaction solution of 5-nitro-N,N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide (formula II) from the ...
Embodiment 2
[0054] Example 2: 1) Add dimethyl 5-nitroisophthalate (850g), aminoglycerin (680g), and anhydrous methanol (680g) into a 5L three-necked glass bottle, and stir for 15 minutes. The temperature of the reaction solution was controlled at 20° C. to 30° C., and sodium methoxide methanol solution (68 g) was added slowly. The temperature of the heating reaction solution is controlled at 30°C to 40°C, and the reaction is stirred for 3 to 4 hours. After the reaction is finished, the temperature of the reaction solution is lowered to 15°C to 25°C, and the temperature of the reaction solution is controlled below 30°C and glacial acetic acid is added until the pH=5.0 to 5.5, and then the reaction is completed. The 5-nitro-N,N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide (Formula II) reaction solution was obtained, which was used for the next step of production.
[0055] 2) Add the reaction solution of 5-nitro-N,N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide (formula II) from ...
Embodiment 3
[0061] Example 3: 1) Add dimethyl 5-nitroisophthalate (850g), aminoglycerin (680g), and anhydrous methanol (680g) into a 5L three-necked glass bottle, and stir for 15 minutes. The temperature of the reaction solution was controlled at 20° C. to 30° C., and sodium methoxide methanol solution (68 g) was added slowly. The temperature of the heating reaction solution is controlled at 30°C to 40°C, and the reaction is stirred for 3 to 4 hours. After the reaction is finished, the temperature of the reaction solution is lowered to 15°C to 25°C, and the temperature of the reaction solution is controlled below 30°C and glacial acetic acid is added until the pH=5.0 to 5.5, and then the reaction is completed. The 5-nitro-N,N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide (Formula II) reaction solution was obtained, which was used for the next step of production.
[0062] 2) Add the reaction solution of 5-nitro-N,N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide (formula II) from ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com