Method for preparing battery-grade cobalt sulfate and cobalt chloride by acid dissolution of cobalt beans and cobalt plates
A battery-grade, cobalt plate acid technology, applied in cobalt sulfate, chemical instruments and methods, cobalt halide, etc., can solve the problems of excessive iron content and high acidity, and achieve the effect of strong applicability, low cost and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
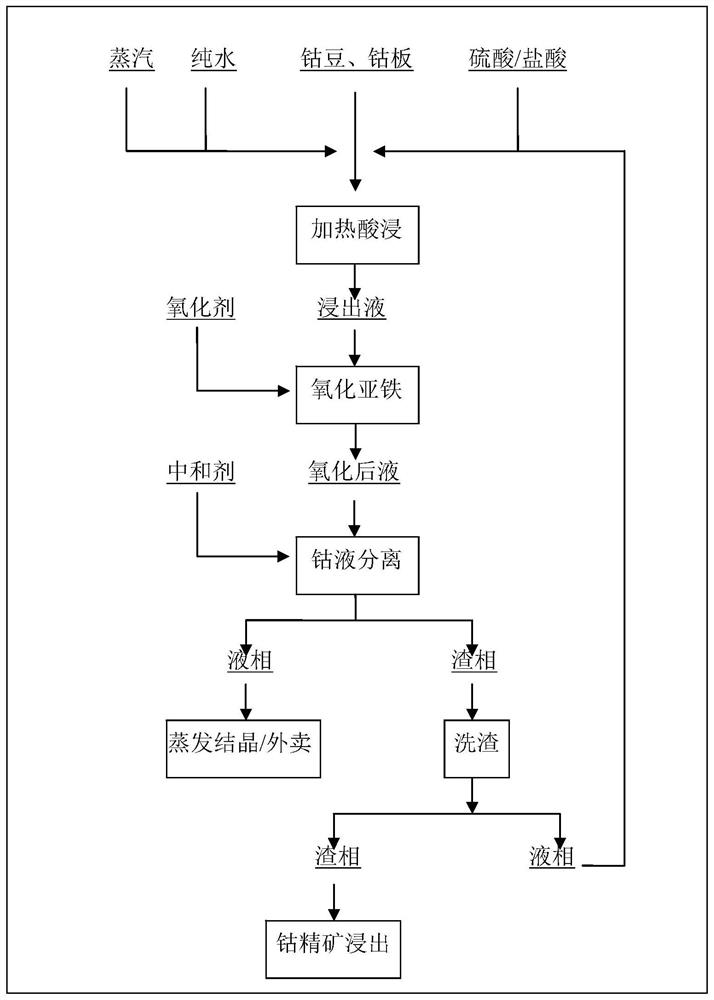
[0037] The raw material is cobalt beans. The leaching solution of cobalt beans leached with heated sulfuric acid contains: 131.0g / L of Co 2+ , 0.52g / L of Fe 2 + , H of 0.61 + .
[0038] Add hydrogen peroxide as an oxidant to the above leaching solution, and the amount of hydrogen peroxide introduced is Fe 2+ 2.8 times the total number of moles, the hydrogen peroxide solution is added to the leaching solution from the middle of the reactor through a metering pump. The reaction temperature is 45°C, the reaction time is 1h, and the reaction is carried out under normal pressure. Then add ammonium bicarbonate to the leaching solution to neutralize H + , the added amount is H + 0.98 times the total number of moles. After that, the solid-liquid is densely separated, the slag phase is sent to the slag washing process, the washing water is returned to the first step as leaching bottom water, and the liquid phase is sent to MVR crystallization or takeaway.
[0039] The cobalt l...
Embodiment 2
[0041] The raw material is cobalt beans. The cobalt bean leaching solution leached with heated sulfuric acid contains: 127.5g / L Co 2+ , 0.44g / L of Fe 2 + , 0.52N H + .
[0042] Add hydrogen peroxide as an oxidant to the above leaching solution, and the amount of hydrogen peroxide introduced is Fe 2+ 2.4 times the total number of moles, the hydrogen peroxide solution is added to the leaching solution from the middle of the reactor through a metering pump. The reaction temperature is 58° C., the reaction time is 1 h, and the reaction is carried out under normal pressure. Then add ammonia water to the leaching solution to neutralize the H + , the added amount is H + 0.99 times the total number of moles. After that, the solid-liquid is densely separated, the slag phase is sent to the slag washing process, the washing water is returned to the first step as leaching bottom water, and the liquid phase is sent to MVR crystallization or takeaway.
[0043] The cobalt liquid obt...
Embodiment 3
[0045] The raw material is cobalt beans. The leaching solution of cobalt beans leached with heated sulfuric acid contains: 133.8g / L of Co 2+ , 0.27g / L Fe 2 + , 0.48N H + .
[0046] Add hydrogen peroxide as an oxidant to the above leaching solution, and the amount of hydrogen peroxide introduced is Fe 2+ 2.5 times the total number of moles, the hydrogen peroxide solution is added to the leaching solution from the middle of the reactor through a metering pump. The reaction temperature is 50°C, the reaction time is 1h, and the reaction is carried out under normal pressure. Then add urea to the leaching solution to neutralize the H + , the added amount is H + 0.48 times the total number of moles. After that, the solid-liquid is densely separated, the slag phase is sent to the slag washing process, the washing water is returned to the first step as leaching bottom water, and the liquid phase is sent to MVR crystallization or takeaway.
[0047] The cobalt liquid obtained by d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com