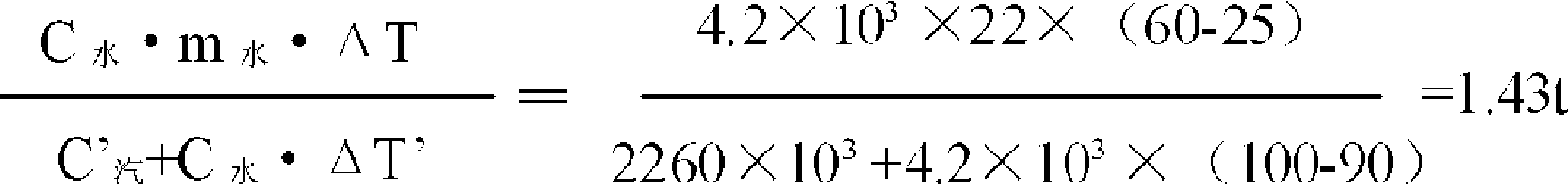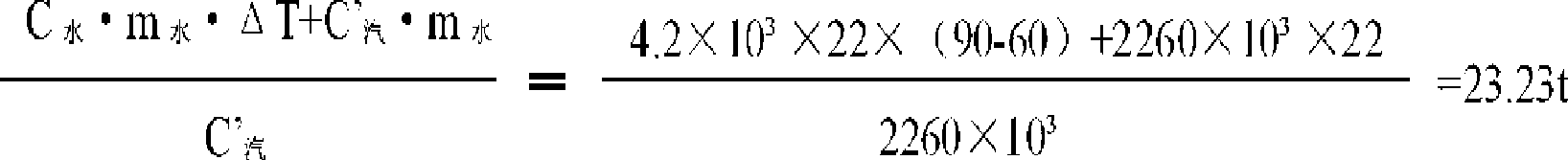Patents
Literature
68results about "Cobalt halides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cathode active material for nonaqueous electrolyte secondary battery and method of producing cathode active material for nonaqueous electrolyte secondary battery
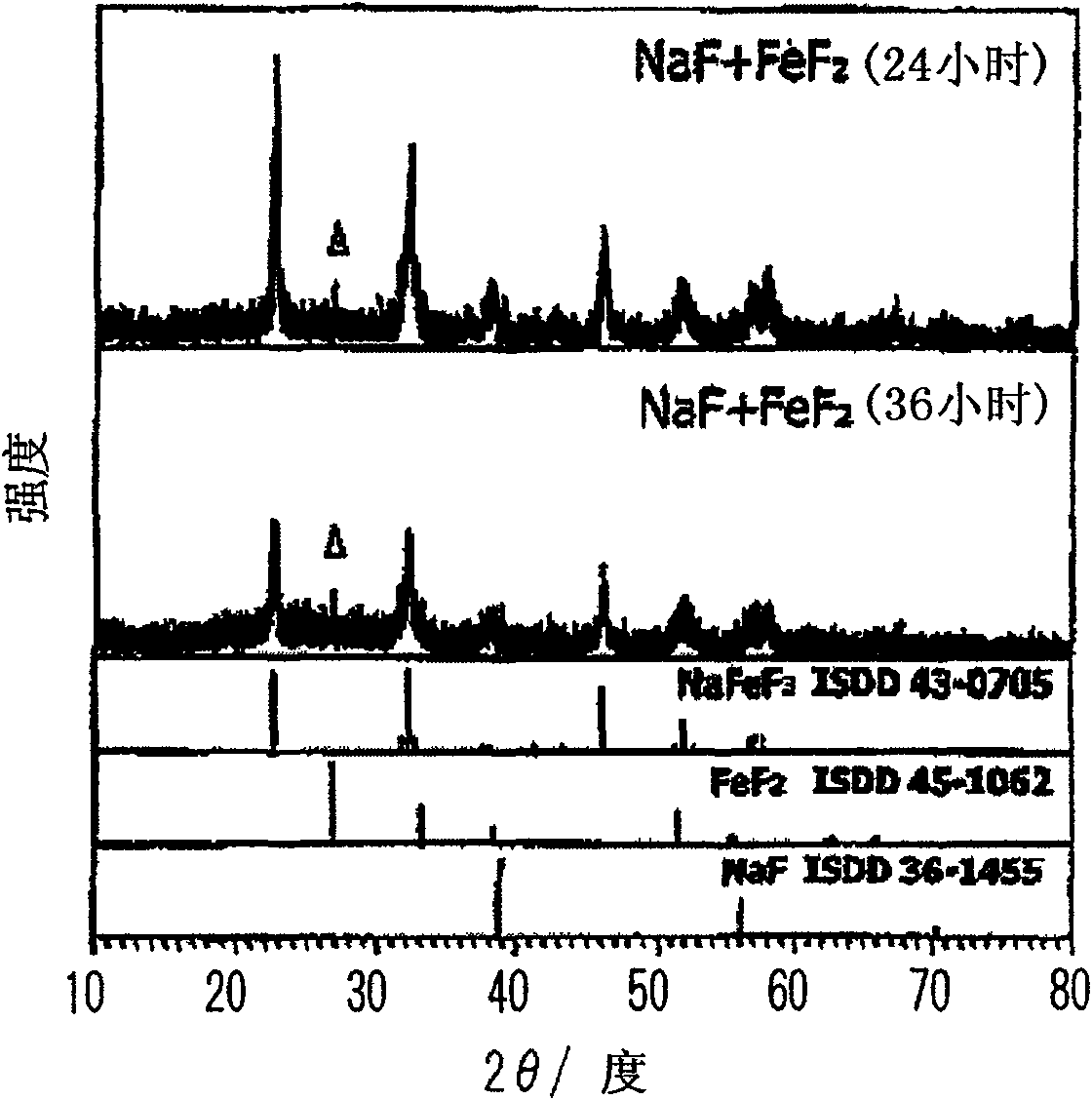
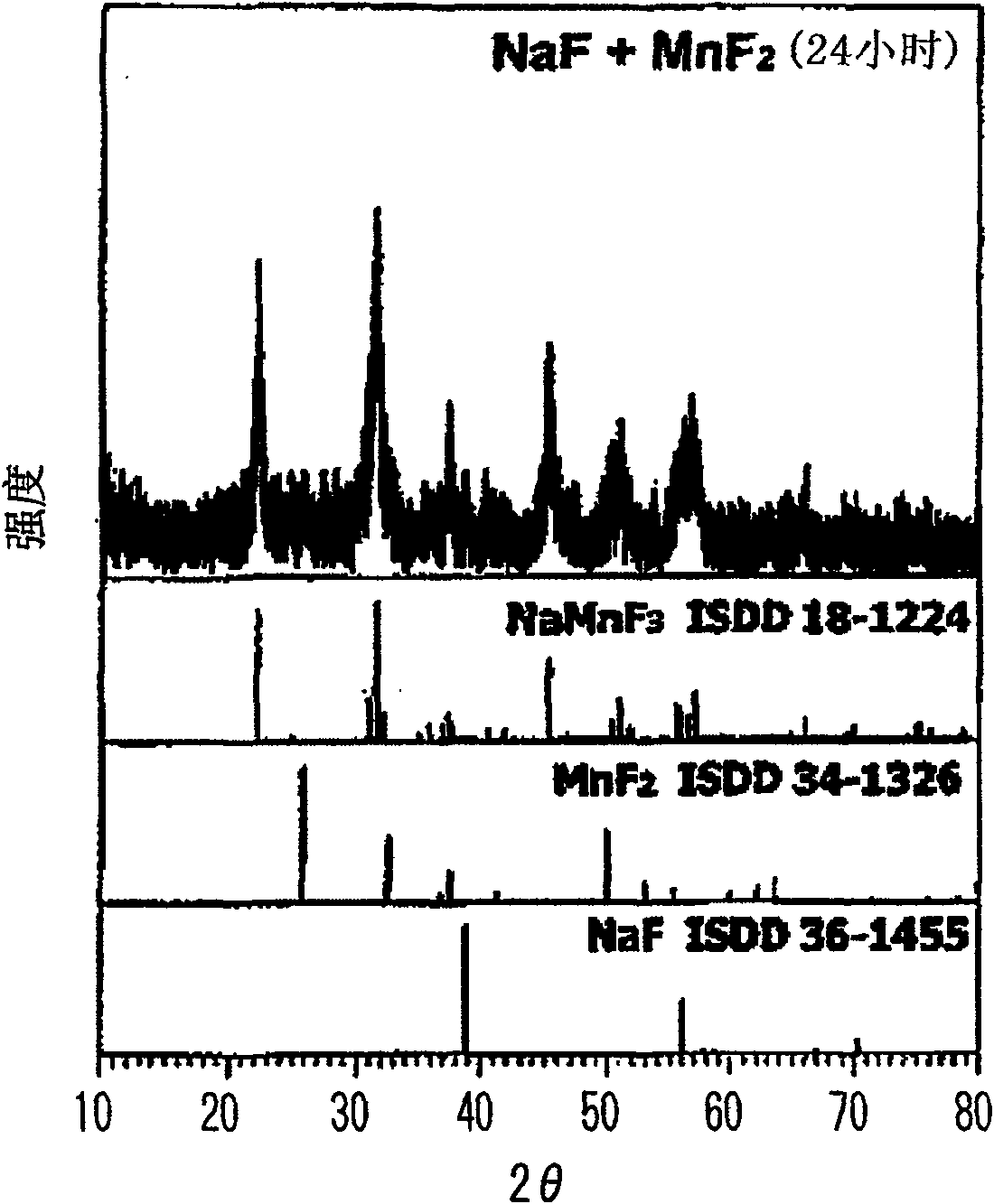
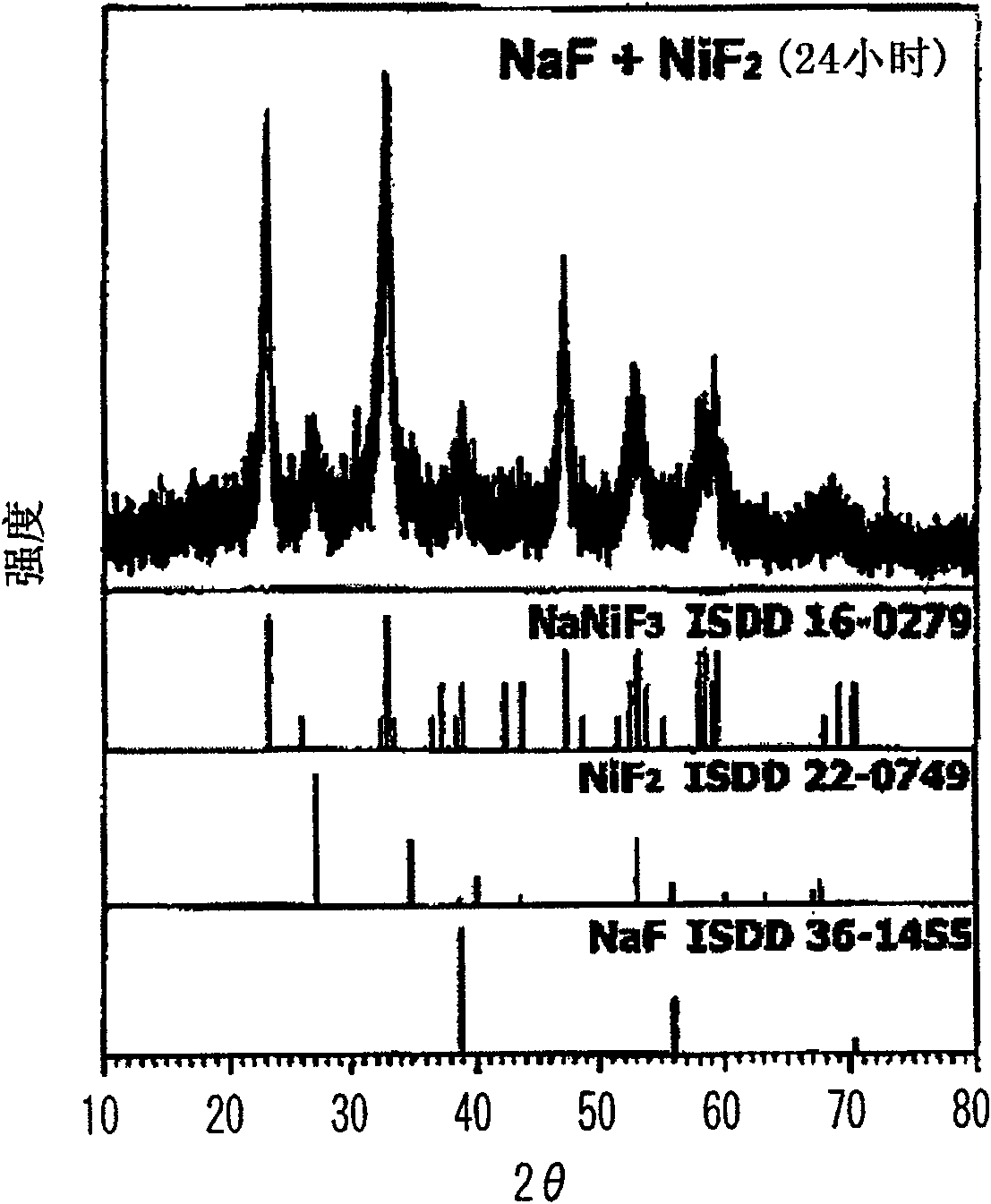
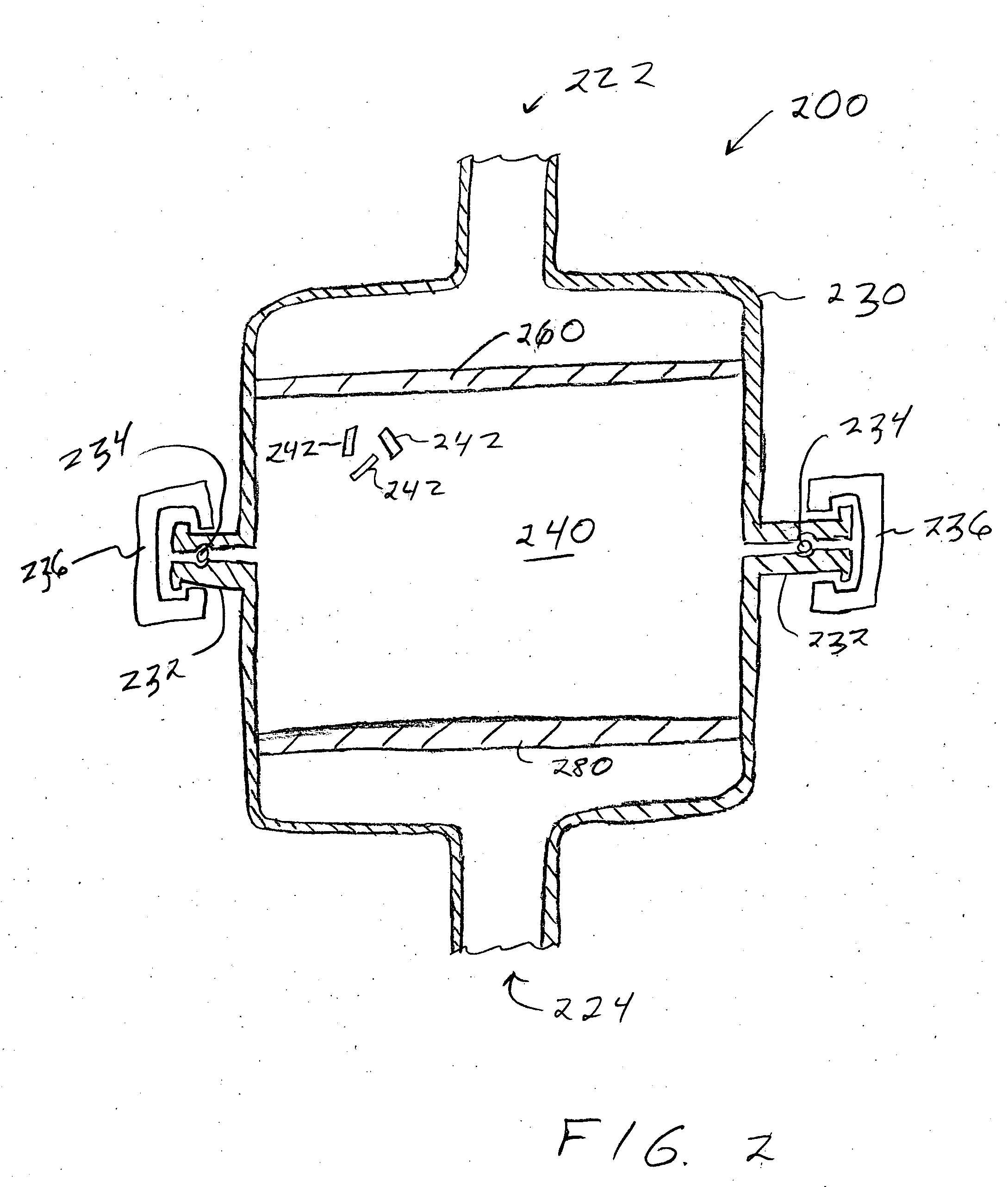
To prepare a fluorinated cathode active material having a guest cation comprising sodium or lithium included therein in a nonaqueous electrolyte secondary battery, an alkali metal fluoride represented by the formula AF and a transition metal fluoride represented by the formula M'F2 are treated by chemical milling to thereby give a fluoride AM'F3. A satellite ball mill is preferably employed in the chemical milling treatment.
Owner:MITSUBISHI HEAVY IND LTD +1
Three-ingredient eutectic ionic liquid and preparation method thereof
InactiveCN103193711ALow costWill not emitZinc halidesUrea derivatives preparationOrganic synthesisToxic material
The invention relates to three-ingredient eutectic ionic liquid and a preparation method thereof. The three-ingredient eutectic ionic liquid consists of ILX, MH and HBD, wherein ILX is ionic liquid with negative-monovalent halogen ions which serve as negative ions, MH is transition metal haloid, and HBD is a compound with multiple hydrogen bonds. The preparation method comprises the following steps of: uniformly mixing ILX, MH and HBD according to the molar ratio of 1: 1: n (n= 0.5-4), heating up to the temperature of 100-140 DEG C, and then, carrying out heat preservation for 2-4 hours until a mixture is completely dissolved. According to the ionic liquid disclosed by the invention, the melting point is extremely low, the viscosity is low, the conductivity is high, the dissolution performance is good, and the ionic liquid has Lewis acidity and catalysis performance, so that the ionic liquid can be widely applied to organic synthesis reaction, the preparation of nano and mesoporous materials, gas absorption and the like; and the preparation process is simple, the raw materials are readily available, the cost is low, no organic solvents are used and no toxic substances are emitted during reaction, no byproducts are produced, and the ionic liquid is biodegradable, so that the ionic liquid is suitable for industrial production.
Owner:DONGHUA UNIV
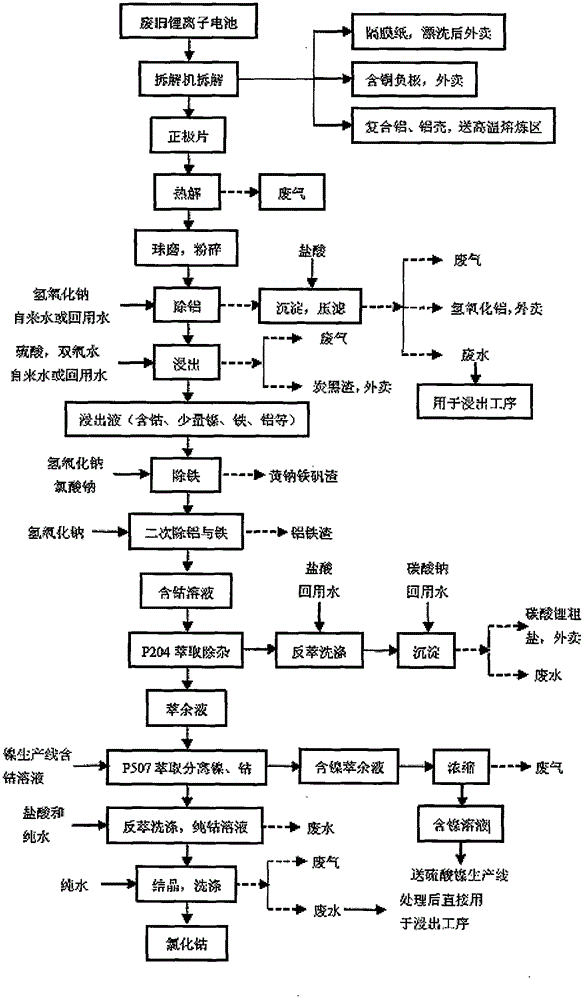
Process for recovering cobalt chloride from waste lithium ion battery
InactiveCN104577247AEfficient recyclingReasonable useCobalt halidesWaste accumulators reclaimingAluminium hydroxideLithium-ion battery
The invention discloses a process for recovering cobalt chloride from a waste lithium ion battery. The process comprises the following steps: dismantling the waste lithium ion battery through a dismantling machine, separating a positive plate, diaphragm paper, a copper-containing negative electrode and an aluminum shell; pyrolyzing and ball-milling the positive plate, adding sodium hydroxide in the ball-milled positive plate, filtering out obtained sediment substance, carrying filter-pressing, adding 1-3mol / l of hydrochloric acid solution to react to obtain aluminum hydroxide, adding one of sulfuric acid or hydrogen peroxide in supernatant to perform leaching reaction; removing iron in leachate, and adding sodium hydroxide to secondarily remove aluminum and iron to obtain a cobalt-containing solution; extracting and removing impurity in the obtained cobalt-containing solution, extracting and separating the obtained extract liquor through P507, re-extracting the obtained cobalt-containing solution to obtain the pure cobalt solution; crystallizing and washing the obtained pure cobalt solution to obtain cobalt chloride. According to the process disclosed by the invention, the operation is simple, the use is convenient, the useful material in the lithium ion battery can be effectively recovered; and the resource is reasonably utilized.
Owner:陈静
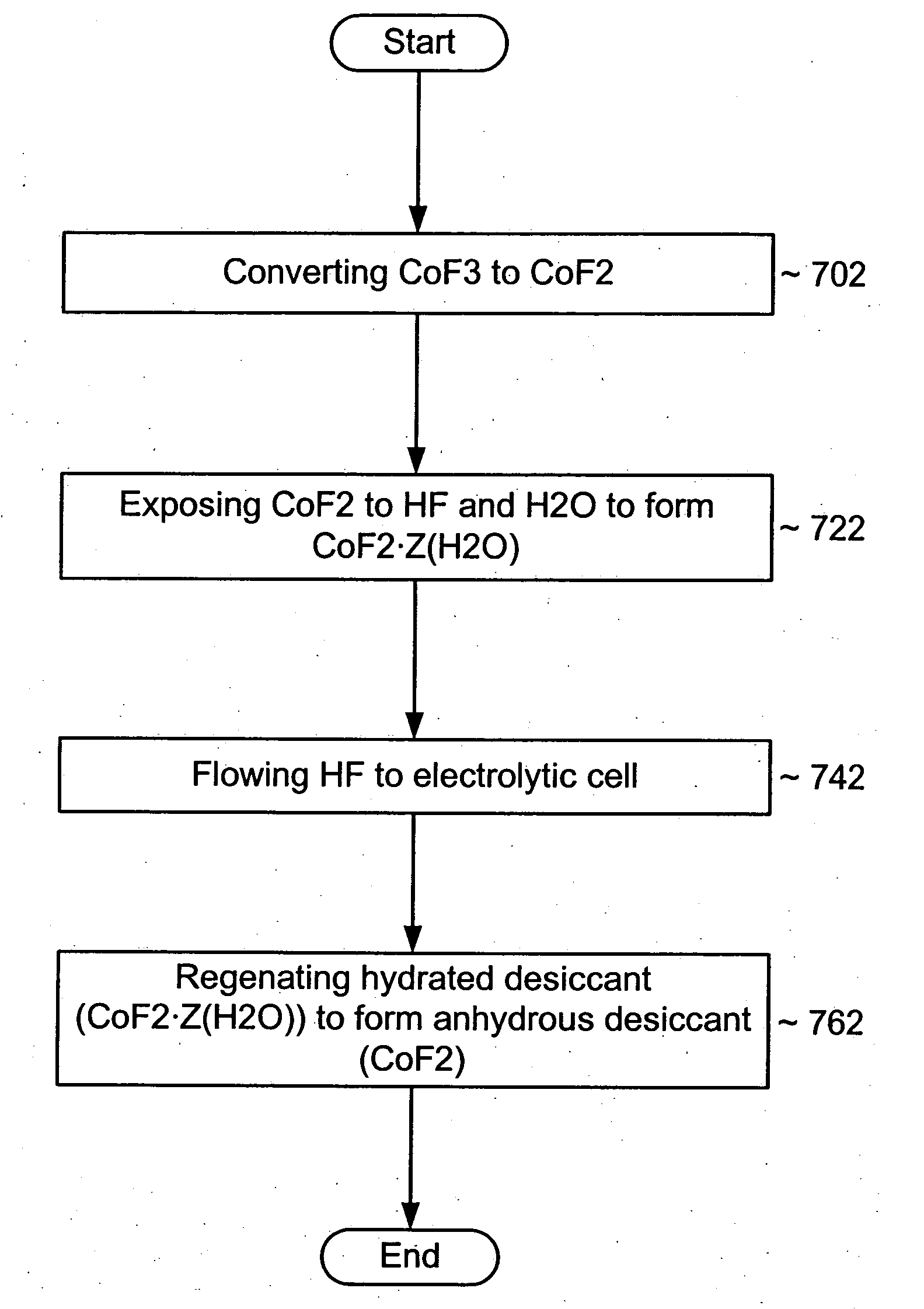
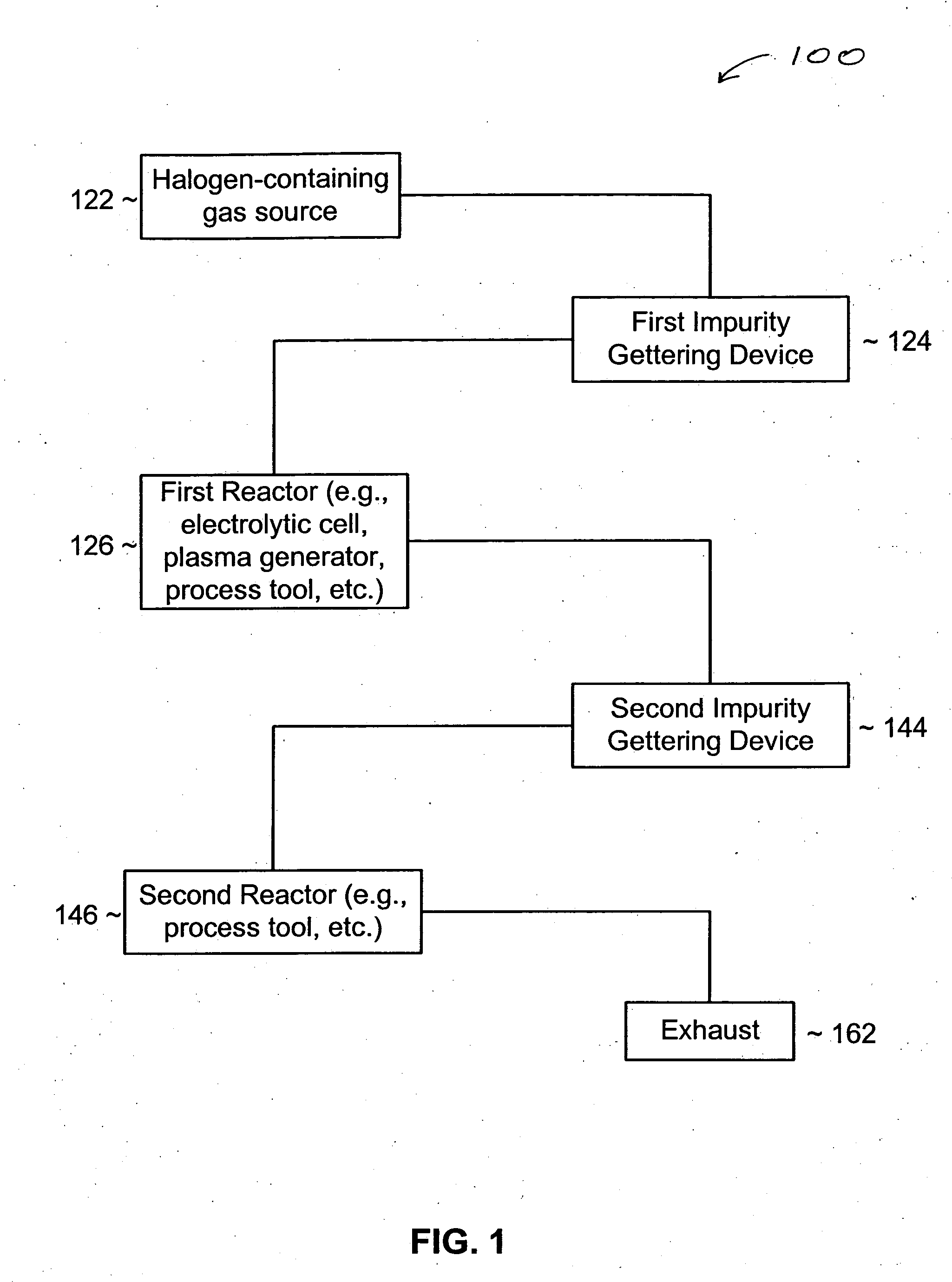
System and process for reducing impurities
InactiveUS20050069475A1Lower the water levelReduce the presence of impuritiesHydrogenPhotography auxillary processesHalogenHydrogen
An impurity gettering device can be installed between a source and a reactor to reduce an impurity from a fluid before it reaches the reactor. More particularly, the impurity gettering device can getter an inorganic, polar, hydrogen-containing impurity (e.g., H2O, NH3, etc.) from a halogen-containing fluid (e.g., a fluorine-containing liquid or gas) by forming ligands to a metal-containing compound to form a complex. In one example, a fluid source may include HF and H2O, which can flow through the impurity getting device that includes COF2. The COF2 can getter the H2O and form CoF2.ZH2O, where Z is an integer. The fluid may become anhydrous HF that can be processed by a reactor, such as an electrolytic cell. By removing H2O before the fluid reaches the electrolytic cell, adverse effects of H2O, such as consumption of a carbon anode, particle generation, etc. can be reduced.
Owner:FLUORINE ON CALL
Porous material and method for producing the same
InactiveCN1798703AExcellent fluorination catalytic abilityHigh porosityMagnesium fluoridesCobalt halidesHydrogen fluoridePhysical chemistry
The present invention provides a raw material composition suitably used for synthesizing a porous metal fluoride having a large surface area and being stable even in a corrosive gas environment or the like. The porous metal fluoride obtained by reacting this raw material composition with anhydrous hydrogen fluoride has a larger surface area and is stable in a corrosive gas environment, etc., and can be used, for example, as a fluorination catalyst.
Owner:权恒道
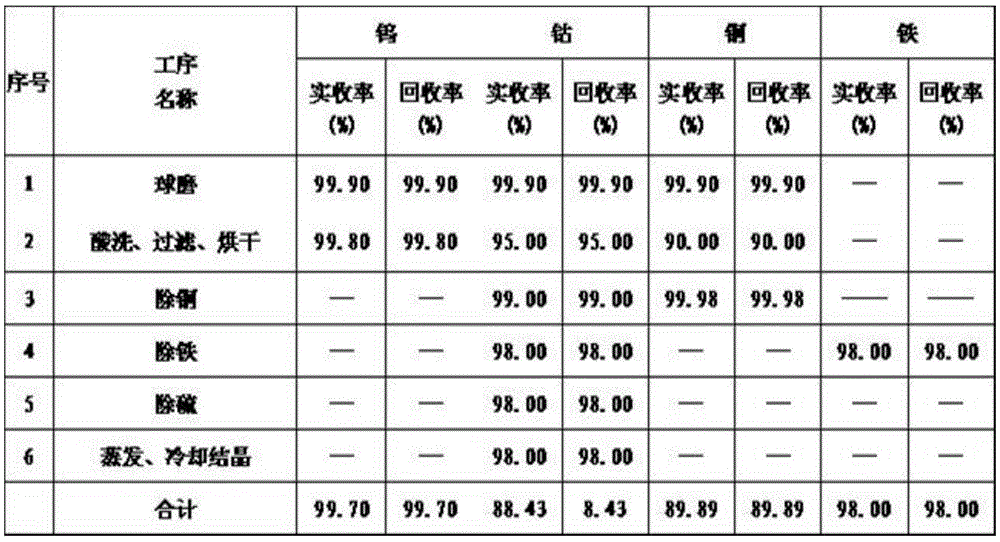
Method for preparing tungsten carbide and cobalt chloride by tungsten-containing waste
ActiveCN105460981AAchieve recyclingAchieving zero emissionsCobalt halidesCalcium/strontium/barium sulfatesMetal impuritiesSlurry
The invention discloses a method for preparing tungsten carbide and cobalt chloride by tungsten-containing waste. The method comprises the following steps: adding water into tungsten-cobalt waste for ball milling; dissolving out metal impurities in the tungsten-cobalt waste with hydrochloric acid; adding water to stir, adding a catalyst and a defoaming agent, and keeping on reacting; performing acid pickling, removing supernatant, performing filter-pressing on lower slurry, washing filter residue tungsten sulfate with clean water for multiple times, and drying; adding sodium carbonate into the pickling solution, adding iron powder, filtering copper residues, adding sodium sulfate, hydrogen peroxide and sodium carbonate into the copper-removed solution to precipitate and separate iron in a mode of sodium jarosite, filtering iron residues, adding barium chloride into the iron-removed solution, precipitating sulfate ions, performing filter-pressing, pumping filtrate into a graphite crucible for evaporating and concentrating, cooling and crystallizing. The method is simple in process and convenient to operate, the tungsten-cobalt waste can be recycled, the tungsten-cobalt recovery rate is high, and energy consumption is low; and the whole process realizes zero emission of waste water, can be used for effectively treating waste gas, and is environmentally friendly.
Owner:HUNAN LITIAN TUNGSTEN IND CO LTD
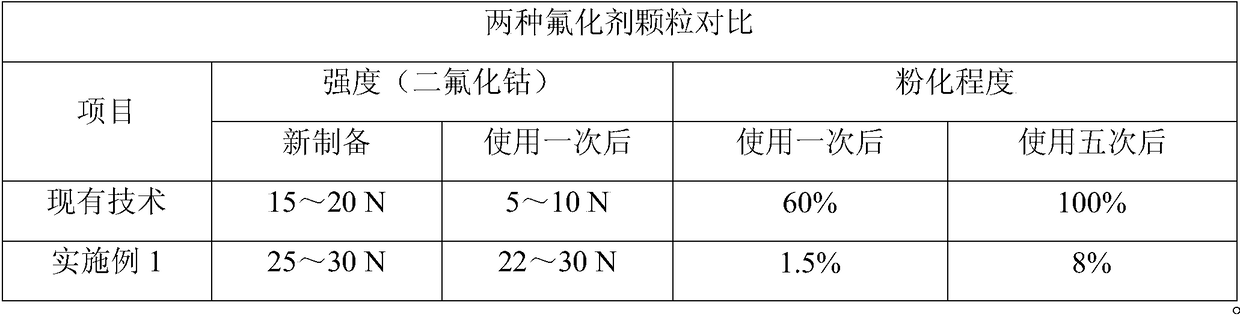
Preparation method of cobaltic fluoride particles
ActiveCN109179517AIntegrity guaranteedSolve the chalking problemCobalt halidesCobalt trifluorideCobaltous chloride
The invention relates to a preparation method of cobaltic fluoride particles, and belongs to the technical field of fluorination catalyst. The preparation method comprises following steps: cobaltous chloride, zinc chloride, aluminium trichloride, and caustic soda are reacted so as to obtain a hydroxide; the hydroxide is subjected to pressing so as to obtain particles, and the particles are subjected to roasting so as to obtain a mixture of cobalt oxide, zinc oxide, and aluminium oxide crystals which is a fluorating agent precursor; the fluorating agent precursor is reacted with anhydrous hydrogen fluoride, and then is reacted with fluorine gas so as to obtain the cobaltic fluoride particles. The fluorating agent precursor extremely high in mechanical strength and stability is prepared firstly, and then the cobaltic fluoride particles are prepared, so that cobaltous fluoride efflorescence problem is solved, cobaltous fluoride is converted into cobaltic fluoride effectively, and cobalticfluoride fluorination efficiency is high.
Owner:无锡玖和隆工程科技有限公司
PAFC (polyamuminum ferric chloride) liquid product and preparation method thereof
ActiveCN106395917AShorten the timePrevent precipitationCobalt halidesWater/sewage treatment by flocculation/precipitationAluminium chlorideHigh concentration
The invention provides a PAFC (polyamuminum ferric chloride) liquid product and a preparation method thereof. According to the preparation method of the PAFC liquid product, during stirring, a sodium hydroxide solution is added to an aluminum chloride and ferric chloride mixed solution in a spray form, precipitate caused by excessive high local pH can be effectively avoided, the time required for preparation of the PAFC liquid product is greatly shortened, meanwhile, the PAFC liquid product with high concentration can be prepared from reaction raw materials with higher concentration, and a large amount of energy can be saved for the following drying procedure. The PAFC liquid product has higher concentration and higher basicity than those of conventional PAFC liquid products in the prior art and can meet higher use requirements as a liquid product, and a large amount of energy can be saved for the following drying procedure in production of solid products.
Owner:新疆澄润环保科技有限公司
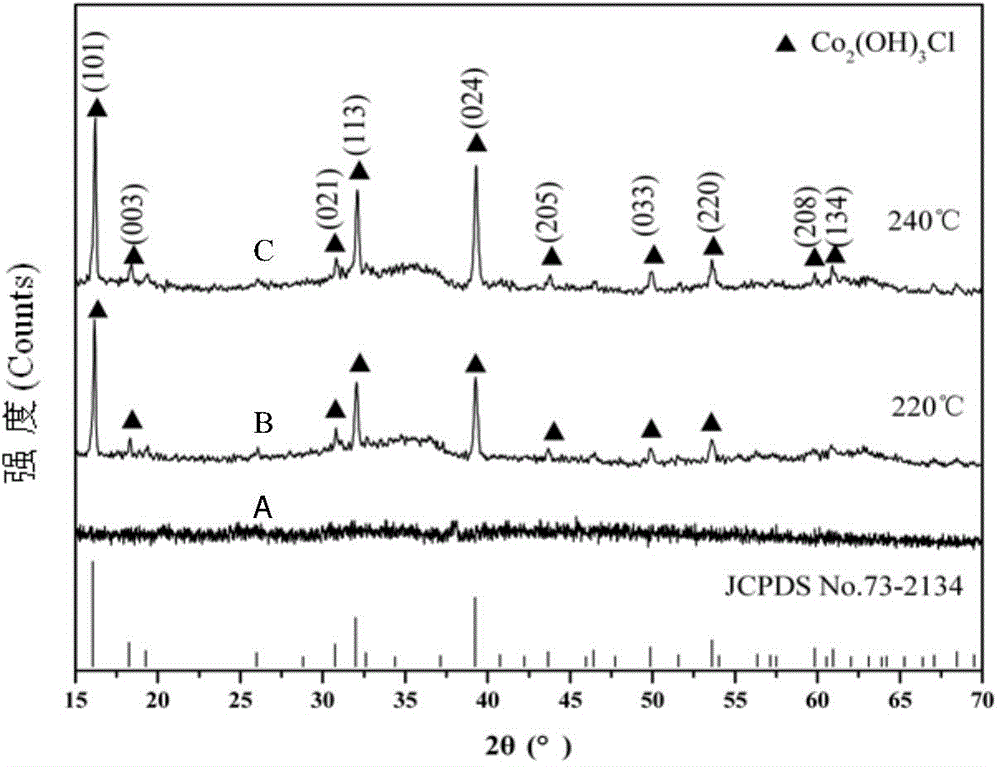
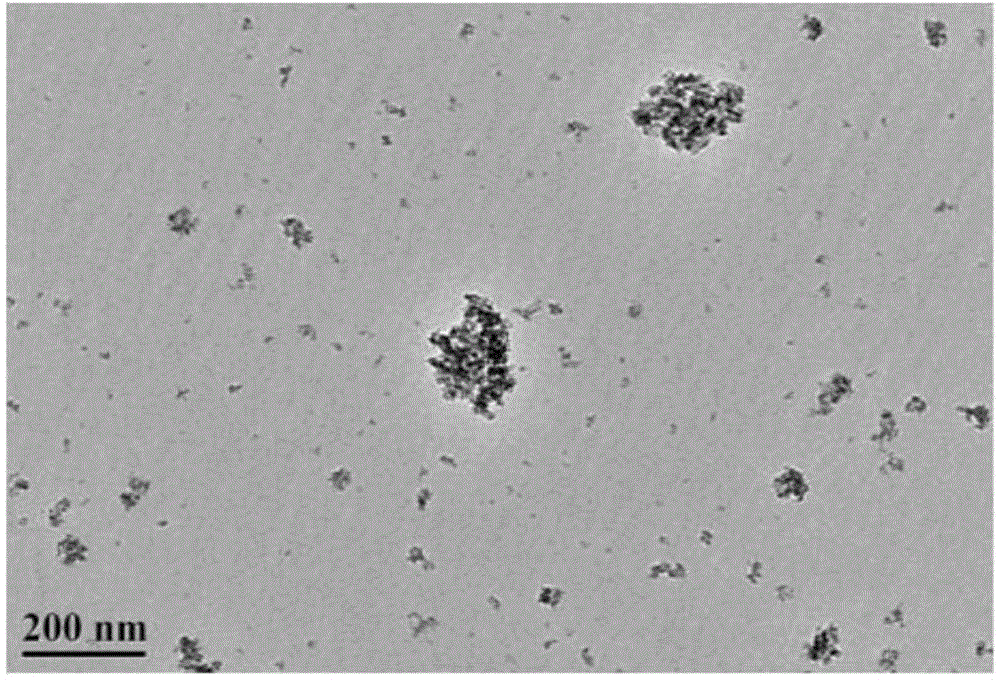
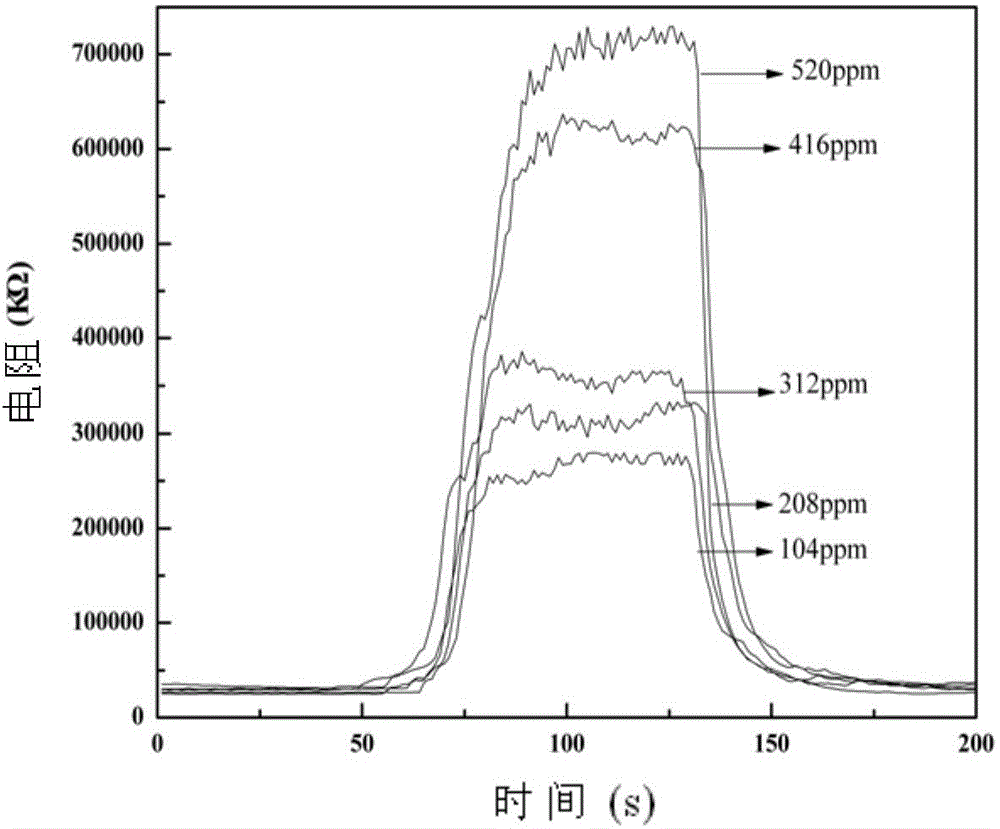
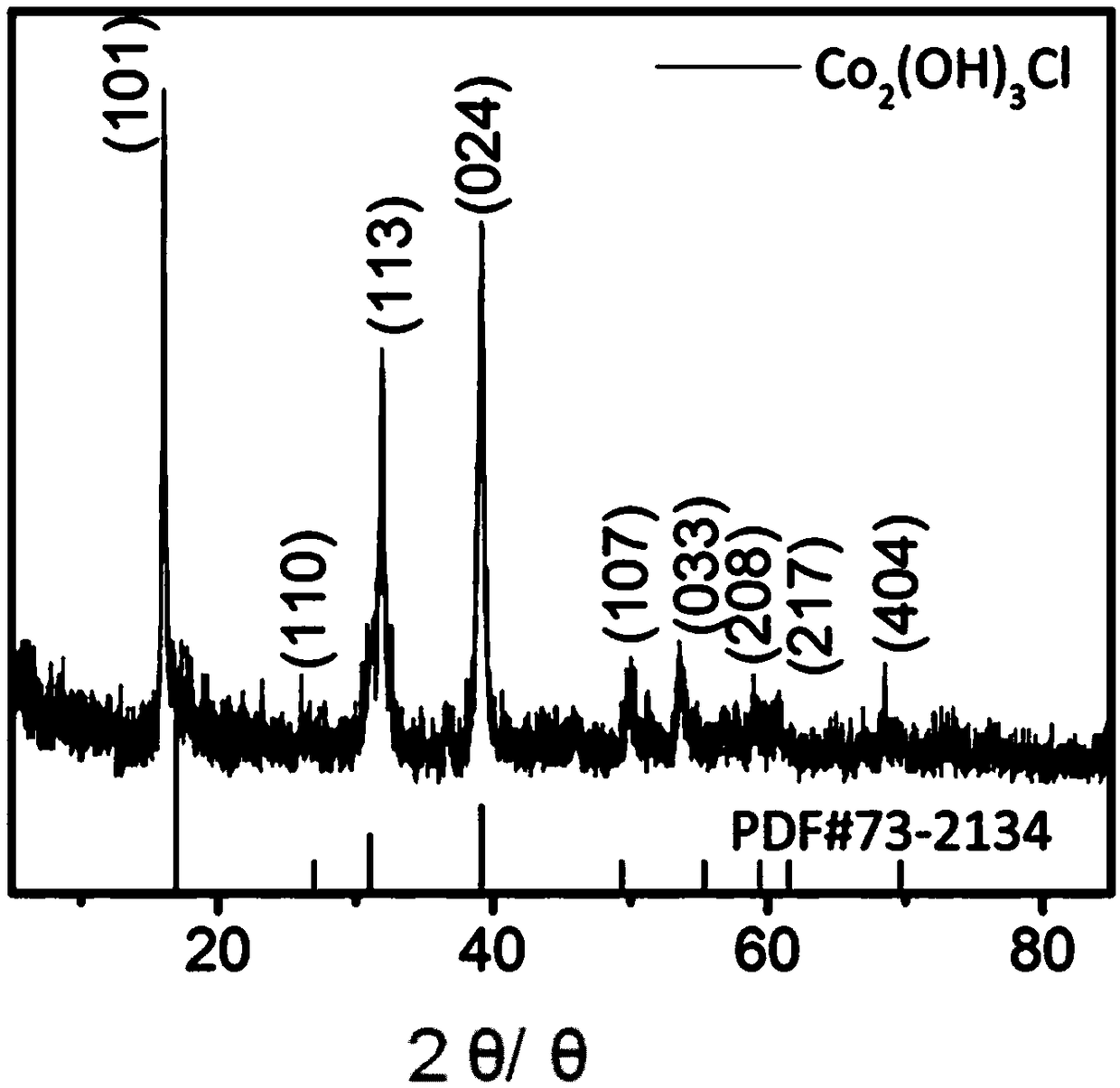
Method for preparing nano Co2(OH)3Cl gas sensitive material by ultrasonic-microwave hydrothermal method and application
InactiveCN104528841ASmall granularityUniform particle sizeMaterial nanotechnologyCobalt halidesCobalt acetateMicrowave
The invention discloses a method for preparing a nano Co2(OH)3Cl gas sensitive material by an ultrasonic-microwave hydrothermal method. The method comprises the following steps: dissolving cobalt acetate tetrahydrate into deionized water to prepare a solution A; adding A TiCl3 hydrochloric acid solution to the solution A, and then carrying out ultrasonic pretreatment to obtain a precursor B; adjusting the pH value of the precursor B to 3.0-11.0; adding to a reaction kettle, reacting under the microwave hydrothermal condition; after reaction is ended, centrifugally collecting a product in the reaction system obtained from the reaction kettle and washing, thereby obtaining the nano Co2(OH)3Cl gas sensitive material. The nano Co2(OH)3Cl gas sensitive material prepared by the method is applied to fabrication of a gas sensitive element, so that the method is simple in process, low in energy consumption and high in synthetic efficiency; and the prepared material is small in particle size and uniform in distribution, and can be applied to fabrication of the gas sensitive element.
Owner:SHAANXI UNIV OF SCI & TECH
Process for producing nickel-cobalt salt and ammonium salt through continuous crystallization
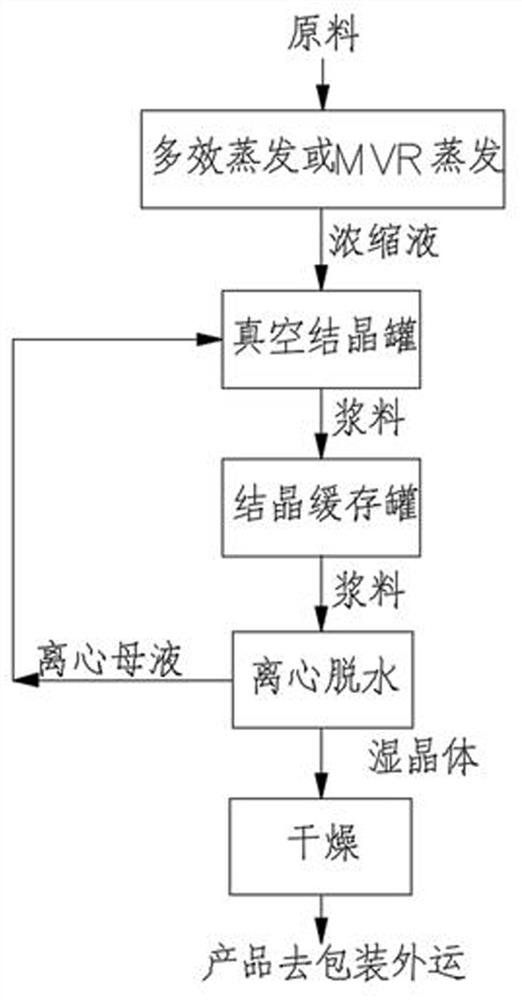
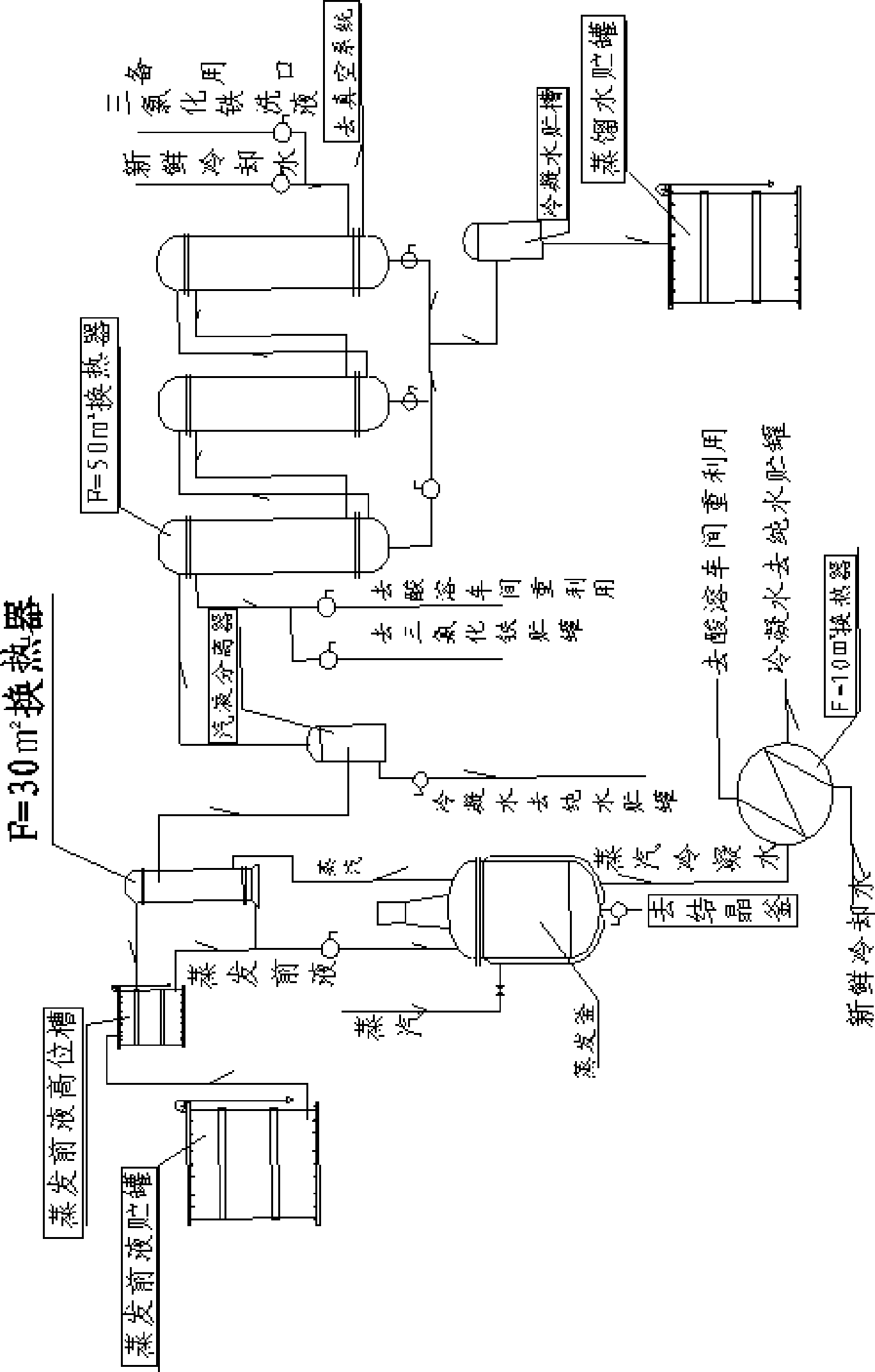
A process for producing nickel-cobalt salt and ammonium salt through continuous crystallization comprises the following steps: a salt-containing solution is concentrated to be close to a saturated state through a multi-effect evaporation device or an MVR evaporation device, the concentrated salt-containing solution enters a vacuum crystallization tank and then is subjected to flash evaporation cooling, salt crystals are separated out while feed liquid circulates in the vacuum crystallization tank, and slurry is obtained; then the secondary steam generated by the vacuum crystallizing tank exchanges heat with circulating cooling water or chilled water through a surface air cooler to be cooled to obtain condensate water and a small amount of non-condensable steam, and the non-condensable steam is pumped out through a vacuum pump or a steam jet pump; finally, the slurry obtained by the vacuum crystallization tank is pumped to the crystallization buffer tank and then sent to the centrifugalmachine by the crystallization buffer tank, and crystals obtained by centrifugal dehydration are dried by the drying bed to obtain crystal products with uniform granularity.
Owner:CHINA CEC ENG
Steam application technological process in the process for preparing the crystal cobalt chloride by cobalt chloride solution
InactiveCN101543685AReduce manufacturing costReduce pollutionCobalt halidesEvaporationPlate heat exchangerOutfall
The invention relates to a steam application technological process in the process for preparing the crystal cobalt chloride by cobalt chloride solution. A first heat exchanger, a second heat exchanger, a set of triple heat exchanger composed of three heat exchangers and a vacuum cooling system are added in the prior process. The steam is utilized at maximum extent, the enterprise production cost is saved and the outfall steam pollution is decreased.
Owner:南通新玮镍钴科技发展有限公司
Method for preparing reagent cobalt chloride hexahydrate
The invention provides a method for preparing a reagent cobalt chloride hexahydrate. The method comprises the following steps of: reacting a metal cobalt slice with a concentrated nitric acid so as to generate a cobalt nitrate, directly reducing the cobalt nitrate by using formaldehyde in the condition of the esistence of hydrochloric acid so as to remove the nitrate radical to generate a cobalt chloride solution, filtering, concentrating and cool crystallizing so as to acquire the cobalt chloride hexahydrate. The method of the invention generates no other impurities except the target products, gas and water in the reaction process, and can obtain pure cobalt chloride hexahydrate. The products acquired by using the method meet the analytically pure standard of GB / T 1270-1996 chemical reagent cobalt chloride hexahydrate. The method has the advantages of simple operation, rapid reaction, short producing flow, high production efficiency, mild process condition, easily controlled property and application in mass commercial process.
Owner:GUANGDONG GUANGHUA SCI TECH
Method for preparation of waterless cobaltous chloride
The invention discloses a preparing method of waterless chlorinate and industrial alcaine with low water cobalt chloride (CoCl2.H2O-CoCl2.3H2O), hydrogen gas and chlorine gas as raw material, which comprises the following steps: using high temperature hydrochloride gas generating by burning hydrogen gas and chlorine gas; carrying low chlorinate into pyrolytic reactor; transferring heat with high temperature hydrochloride gas; generating pyrogenic reactor under the catalysis of electromagnetic wave through the pyrolytic reactor; getting waterless chlorinate and water vapor; entering part gas phase material into hydrochloride synthetic furnace through air-solid segregation; proceeding cyclical usage; displacing left gas phase material into hydrochloride recovery system; getting industrial alcaine.
Owner:谷亮
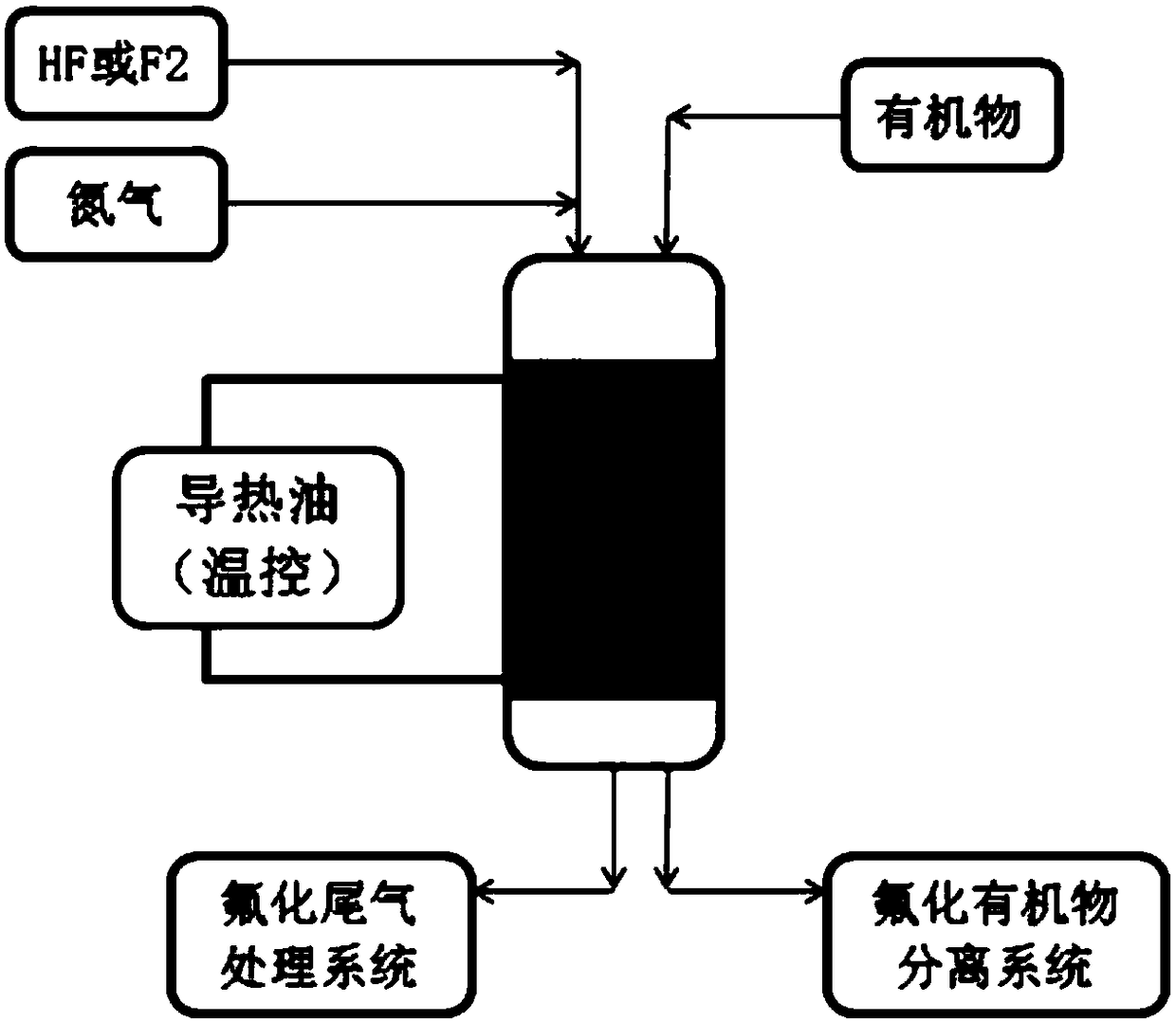
Preparation method of transition metal fluoride
ActiveCN109081383AHigh crystallinityEasy to operateCobalt halidesNickel halidesHigh volume manufacturingPhysical chemistry
The invention discloses a preparation method of a transition metal fluoride, comprising: mixing a transition metal precursor and ammonium fluoride in a mass ratio of 1:(5-20) or placing them with oneupstream and the other downstream, pyrolyzing at 250 DEG C and above, washing with water, filtering, and drying to obtain the transition metal fluoride. The preparation method is simple to perform; afluorine source and transition metal material herein are simple; the cost is low; the transition metal fluoride has good crystallizing performance and is suitable for batch production.
Owner:YANGZHOU UNIV
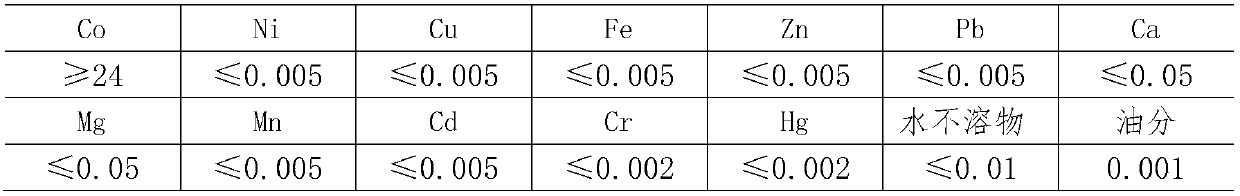
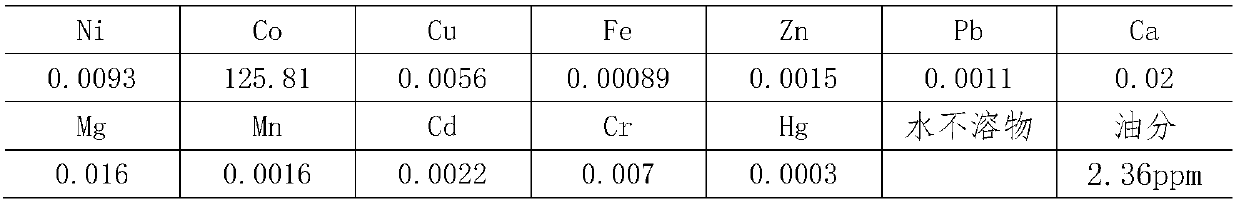
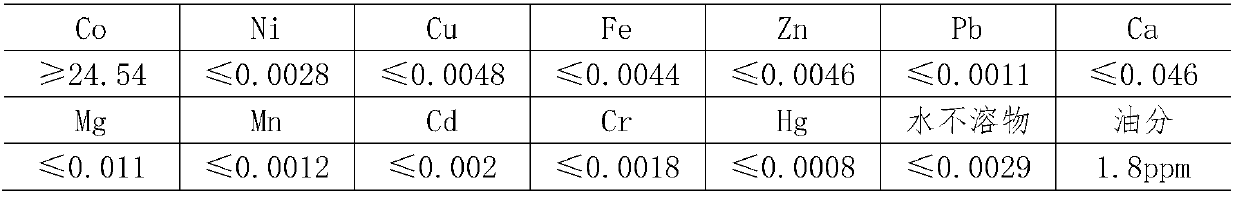
Preparation method of superior pure cobalt chloride
The invention relates to a preparation method of superior pure cobalt chloride. The preparation method comprises the following steps of: (1) reacting metallic cobalt with hydrochloric acid; (2) removing impurities, adding hydrogen peroxide into a cobalt chloride solution obtained in the step (1), oxidizing to remove iron, adjusting the solution by using ammonia water until the PH value is 8-9 so as to ensure that ferric hydroxide precipitates completely, standing for 3-5 hours, and filtering to obtain the solution; and (3) pouring the solution obtained in the step (2) into a glass beaker, adjusting the hydrochloric acid until the PH value is 3-3.5, concentrating at the concentrating temperature of 170-190 DEG C, and when the concentration of the concentrated solution is 38-40 degree / BE, stopping heating, cooling and crystallizing, and centrifuging for separation to obtain the superior pure cobalt chloride. The preparation method comprises the following steps of: reacting metallic cobalt with the hydrochloric, regulating the PH value by adding the hydrogen peroxide and the ammonia water, and removing metal cations, so that precipitation of hydroxide is generated and the superior pure cobalt chloride is prepared.
Owner:TIANJIN CHEM REAGENT RES INST
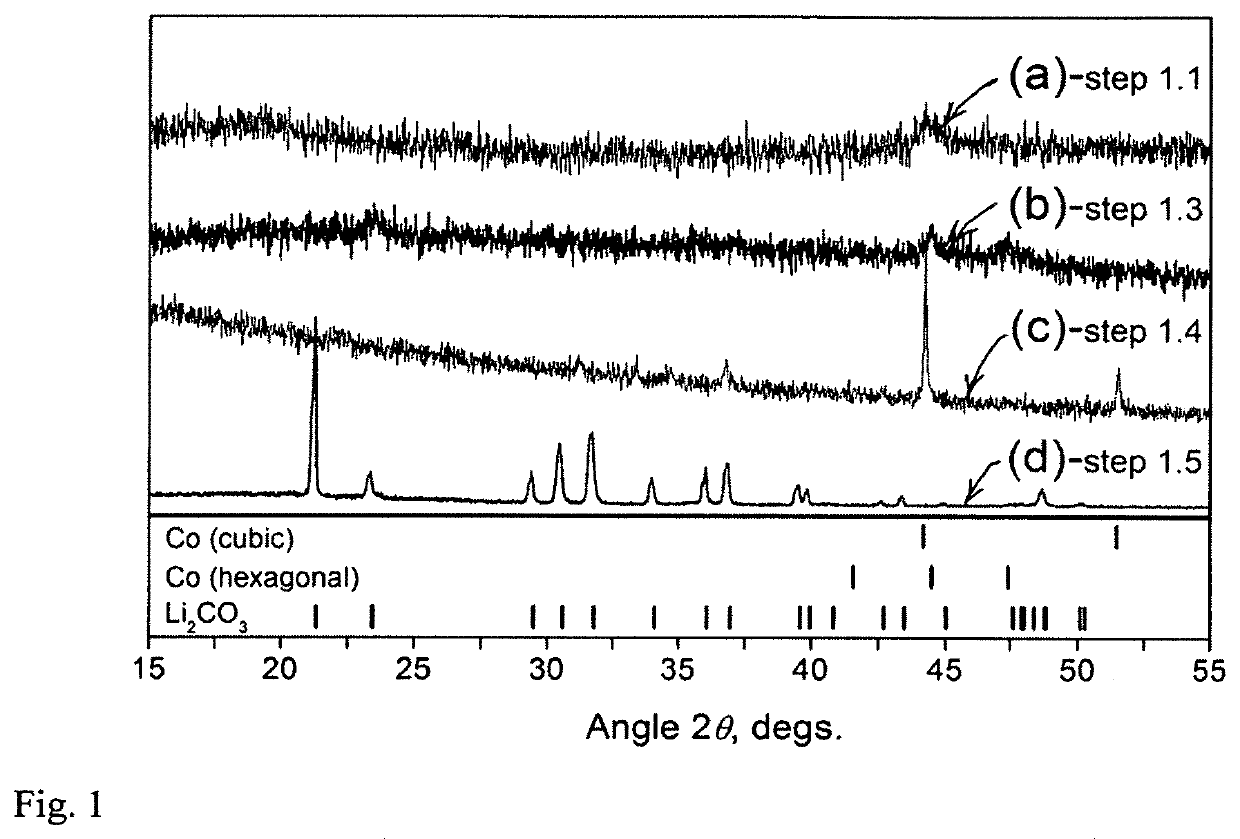
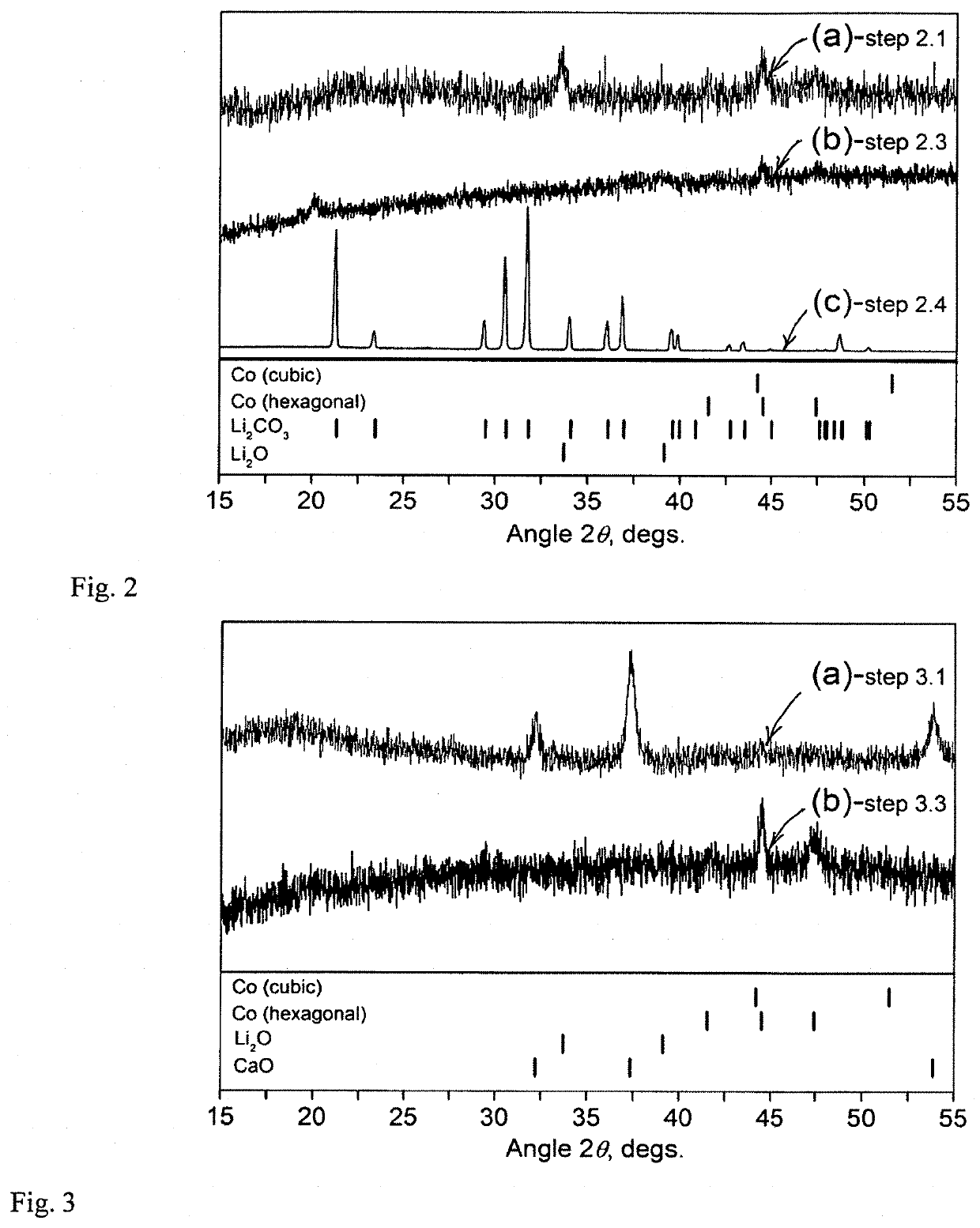
Mechanochemical recovery of Co, Li and other constituents from spent lithium-ion batteries
Method embodiments useful for recycling spent lithium-ion battery (LIB) electrodes to extract critical and / or valuable elements from LIBs are provided and involve mechanochemical processing of spent LIB electrodes in the presence of certain chemical agents to recover products that can include, but are not limited to, metallic solids such as elemental metals or metal alloys, and / or inorganic compounds, metal salts, or organometallic derivatives. The desired products can be separated from by-products and contaminants and further processed into LIB electrode materials or / and other substances.
Owner:IOWA STATE UNIV RES FOUND
Process for the extraction of specific transition metals with gaseous HCL
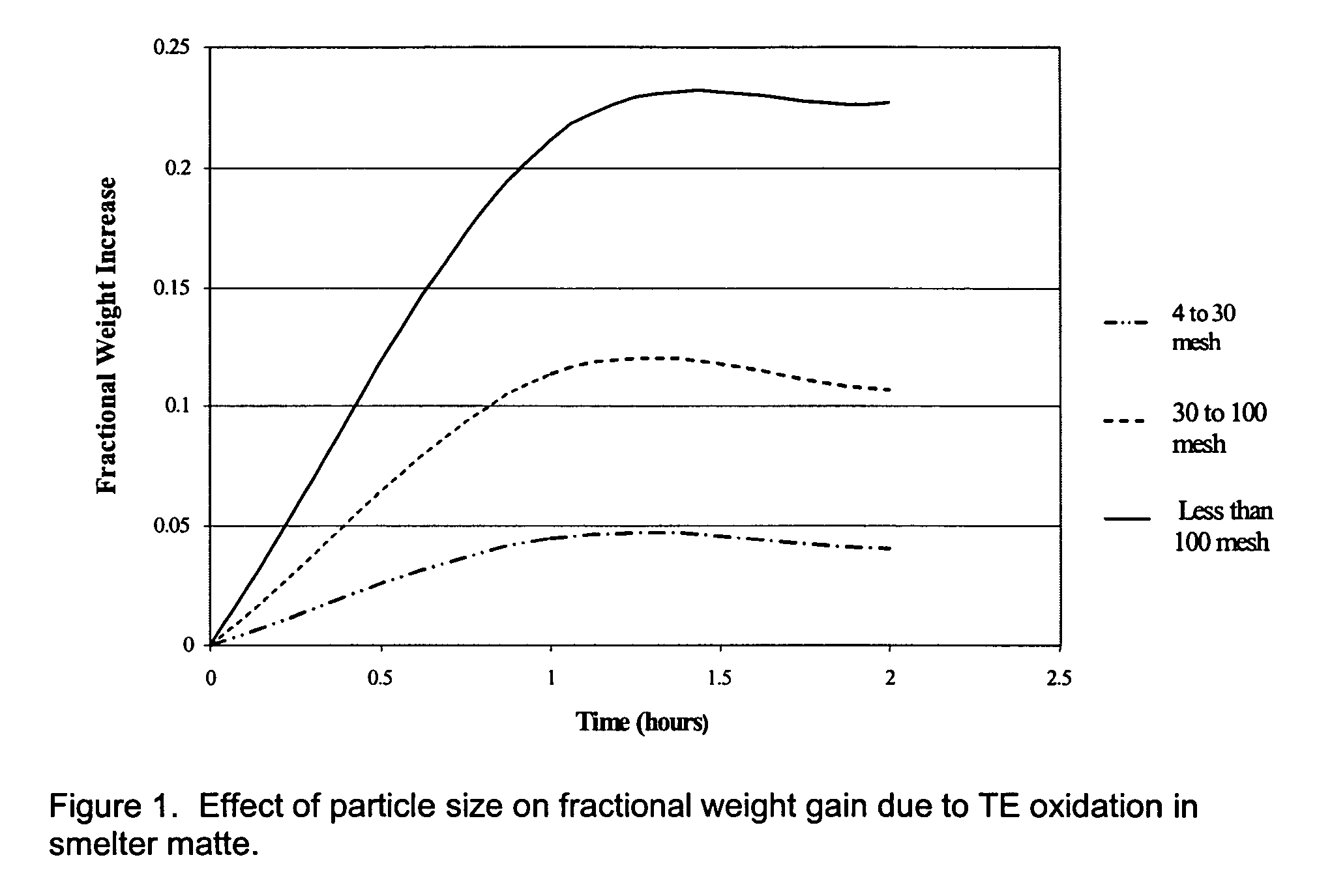
A process is disclosed for separation and recovery of vanadium, molybdenum, iron, tungsten, cobalt and nickel from alumina-based materials, mattes, ores, manufacturing by-products and waste. These elements are oxidized. The oxides are reacted with gaseous HCl to form volatile chloride-bearing compounds that subsequently sublimate. The volatile compounds are condensed in a downward-stepped thermal gradient that allows collection of moderate to high purity compounds of individual elements with exception of a nickel-cobalt co-condensate. Nickel is separated from cobalt by precipitation of nickel chloride from concentrated HCl pressurized with gaseous HCl.
Owner:METALS RECOVERY TECH
Positive active material for lithium secondary battery and method of preparing same
ActiveUS20050181281A1Improved cycle life characteristicsExcellent capacity and potential characteristicPhosphatesFluoride preparationCrystallographyLithium
Disclosed in a positive active material for a lithium secondary battery including a compound represented by formula 1 and having a 10% to 70% ratio of diffracted intensity of diffraction lines in 2θ=53° (104 plane) with respect to diffracted intensity of diffraction lines in the vicinity of 2θ=22° (003 plane) in X-ray diffraction patterns using a CoKα-ray,LixCoO2-yAy (1)wherein, x is from 0.90 to 1.04, y is from 0 to 0.5, and A is selected from the group consisting of F, S and P.
Owner:SAMSUNG SDI CO LTD
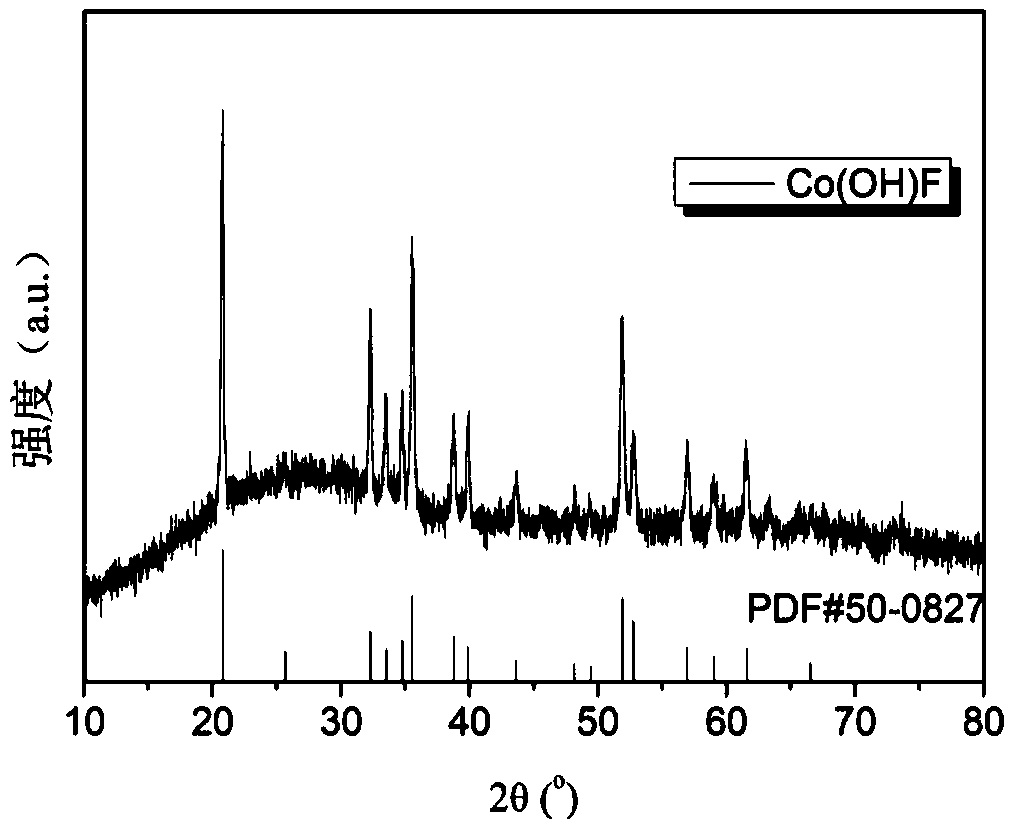
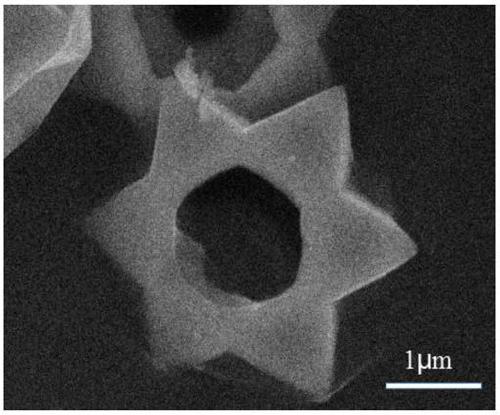
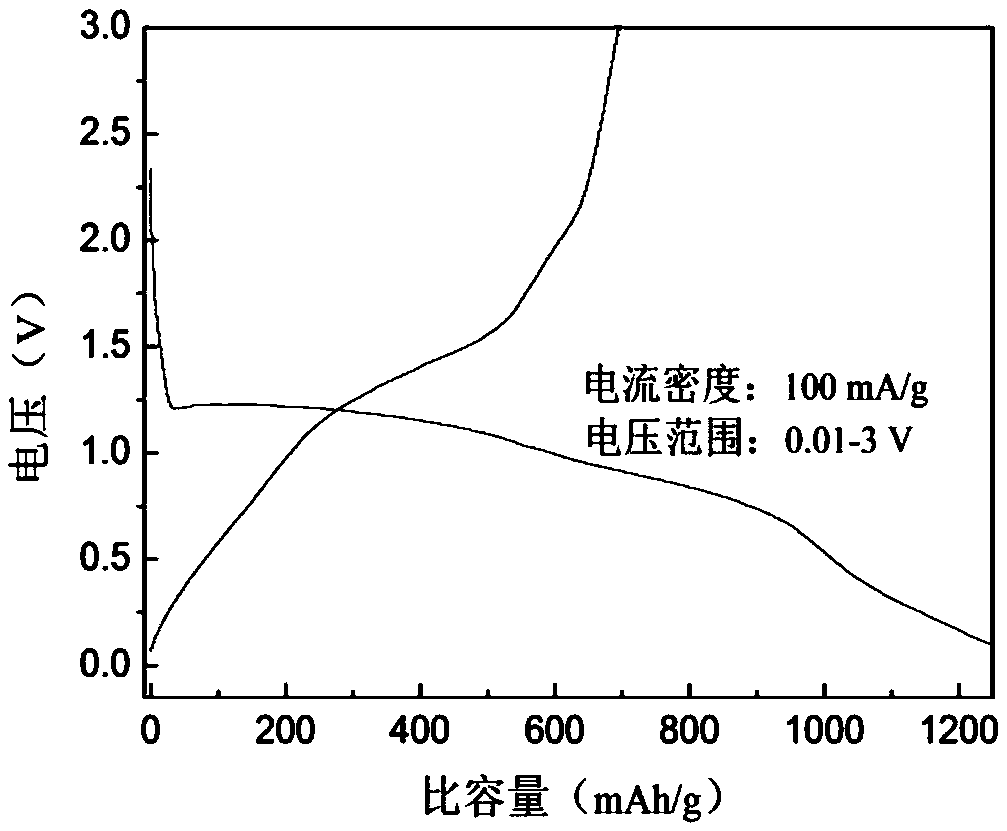
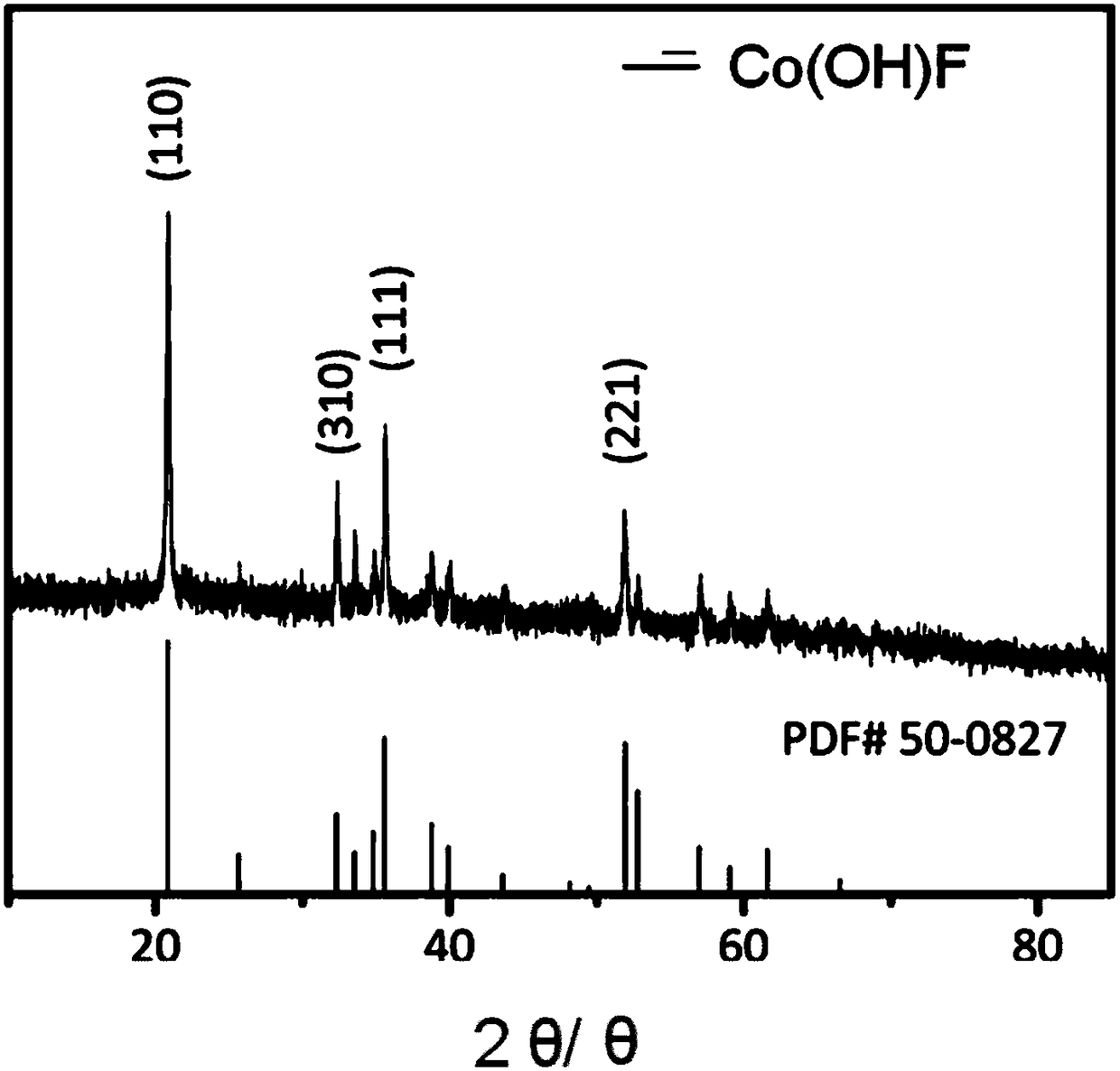
A lithium-ion battery negative electrode material co(oh)f with hexagonal star structure and preparation method thereof
ActiveCN107265518BEasy to operateImprove cycle stabilityCobalt halidesNegative electrodesCyclic processSynthesis methods
The invention belongs to the fields of electrochemistry and new energy and relates to a lithium ion battery negative electrode material Co(OH)F having a hexagram-shaped structure and a preparation method thereof. The lithium ion battery negative electrode material Co(OH)F is synthesized through a solvothermal method. Through adjustment and control of the synthesis technology, solid and hollow hexagram-shaped structure Co(OH)F materials having different morphologies are obtained. The hollow structure can buffer the volume expansion of the electrode material during the circulation so that good circulation stability is kept. The hollow structure is conducive to infiltration of an electrolyte, increasing of the electrode reaction area and improvement of lithium-ion transmission efficiency. The synthesis method is simple in operation, can be carried out under mild conditions, is environment-friendly, has strong repeatability and a short period and is easy to industrialize.
Owner:UNIV OF SCI & TECH BEIJING
Method for producing high-purity cobalt chloride by utilizing cobalt carbonate defective products
ActiveCN108328666AGuaranteed temperatureHigh Purity Cobalt ChlorideCobalt halidesAluminum IonHydrogen
The invention discloses a method for producing high-purity cobalt chloride by utilizing cobalt carbonate defective products. The method comprises the following steps: adding defective cobalt carbonateand water into a reaction kettle and pulping; then adding acid to regulate the pH (Potential of Hydrogen) to sufficiently dissolve the cobalt carbonate; then adding the cobalt carbonate to inverselyregulate the pH; after carrying out filter pressing, obtaining a first cobalt chloride solution; adding the cobalt chloride solution into the reaction kettle capable of being heated and being stirredand improving the temperature to 80 to 90 DEG C; under the condition of sufficiently stirring, slowly adding a sodium hydroxide solution to adjust the pH to ensure that the solution contains enough hydroxyl ions and aluminum ions are sufficiently hydrolyzed; when the pH is completely stable, slowly adding an excessive amount of ammonium fluoride to ensure that magnesium ion sediment is completelyremoved; carrying out heat insulation and stirring; after filtrating, obtaining a high-purity cobalt chloride solution. The method disclosed by the invention has a simple technology and low productioncost; the recovery rate of cobalt reaches 99 percent or more and reaches battery-grade standards.
Owner:XIAMEN TUNGSTEN
Preparation method and use of Cox(OH)yM
InactiveCN108467068ALarge specific surface areaUniform sizeCobalt halidesCell electrodesAlcoholLithium-ion battery
The invention provides a preparation method and use of Cox(OH)yM, wherein M is F or Cl or PO4<3->, CoMa.nH2O is added to an organic alcohol amine solution and stirred until completely dissolved to obtain a mixture; and the mixture is transferred to an autoclave for hydrothermal reaction, and after the end of the reaction, a product is washed and dried to obtain the Cox(OH)yM, wherein M is F or Clor PO4<3->. A low temperature hydrothermal method is adopted in the preparation process, and the steps are simple and easy to operate. The product prepared by the method has large specific surface area, uniform size, and good electrochemical energy storage performance, and can be used as a negative electrode material for high performance lithium ion batteries.
Owner:NINGXIA UNIVERSITY
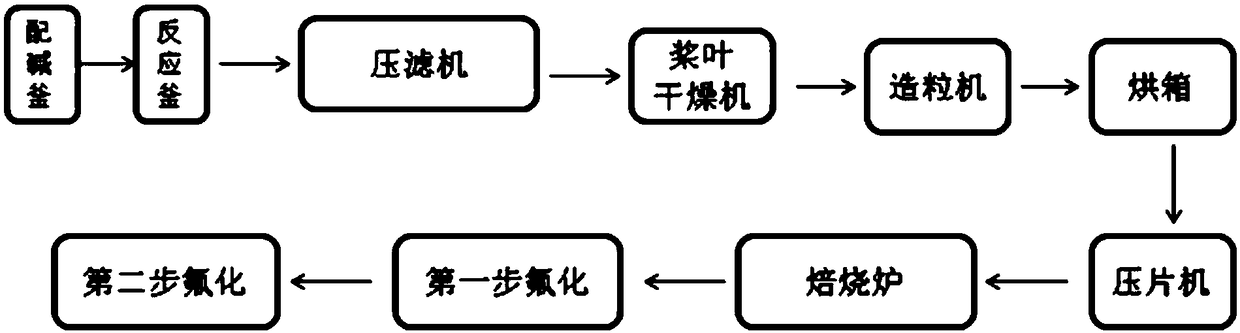
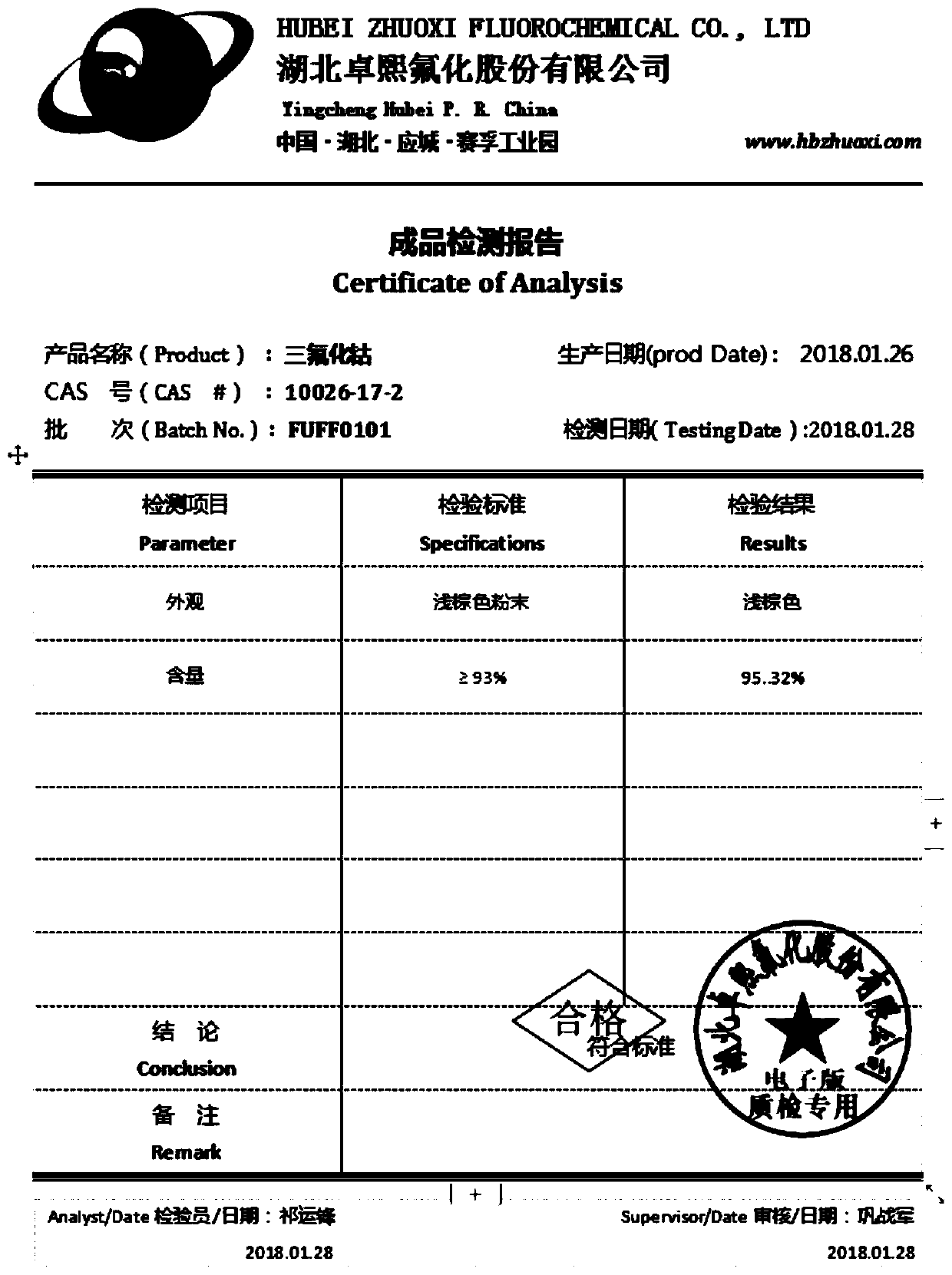
Preparation method of cobalt trifluoride
The invention provides a preparation method of cobalt trifluoride, which comprises the following steps: step 1, mixing cobalt difluoride with ammonium salt in a weight ratio of (20-60):1; 2, feeding the reactants into a reaction kettle, and feeding fluorine gas to carry out fluorination reaction. According to the preparation method, cobalt difluoride and ammonium salt are mixed and then fed into areaction kettle before fluorination production, so that the fluorine consumption is reduced, and the content of cobalt trifluoride in a final product is increased. The content of cobalt trifluoride in the produced cobalt trifluoride product can reach 95.81%, and the content of cobalt difluoride does not exceed 5%.
Owner:HUBEI ZHUOXI FLUOROCHEM
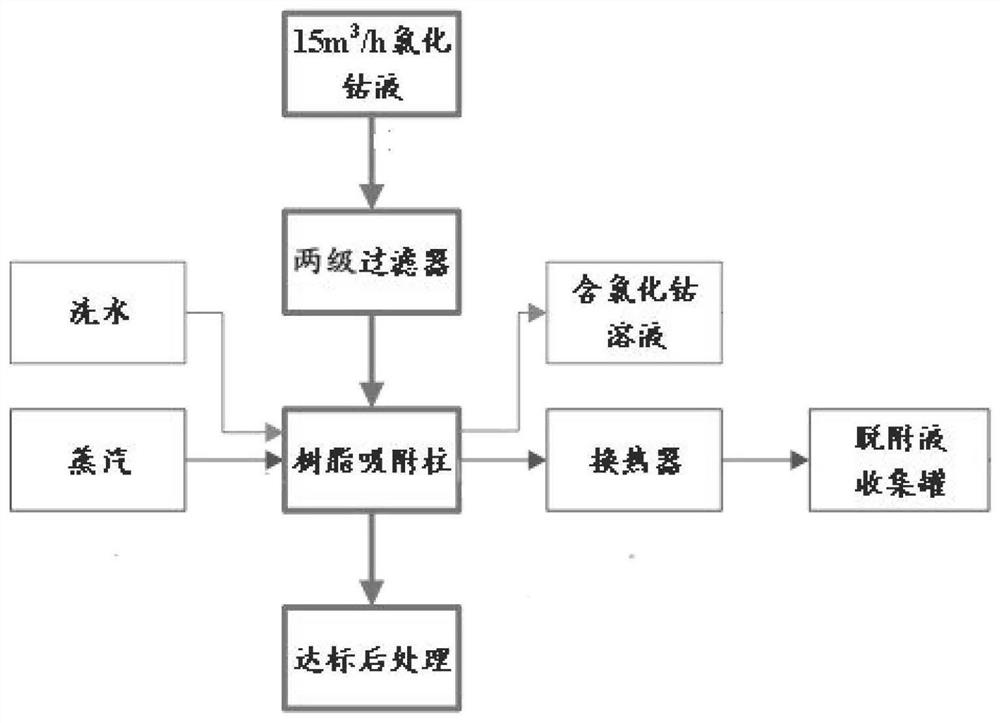
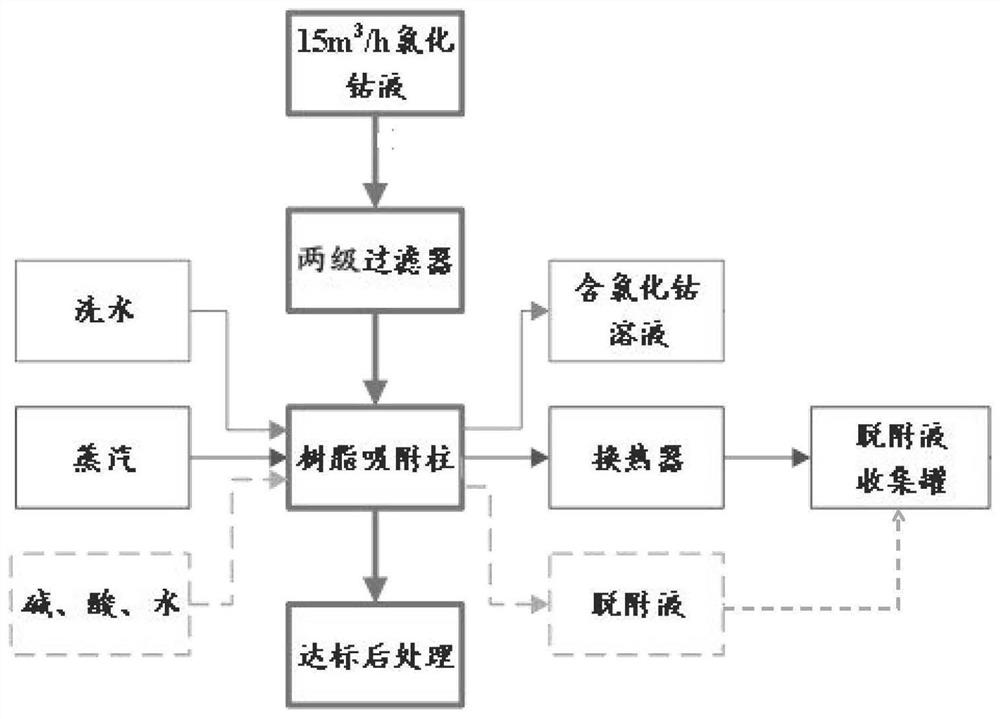
Circulating oil removal method for cobalt chloride solution
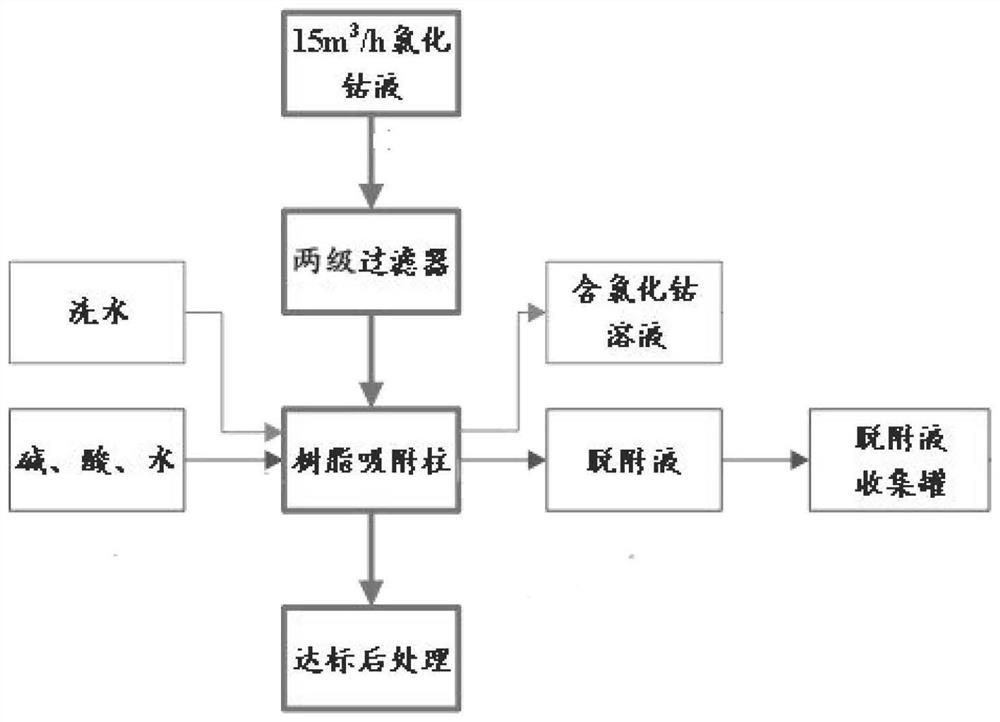
PendingCN114177694AEfficient removalHigh oil removal rateIon-exchange process apparatusElectrolysis componentsPhysical chemistryPolymeric adsorbent
The present invention relates to the technical field of cobalt chloride solution purification, and provides a cobalt chloride solution circulation oil removal method, which comprises: lifting a cobalt chloride solution through a pump, and sequentially passing through a cloth bag filter and a white ball filter; feeding the filtered cobalt chloride solution into a resin adsorption column to obtain a TOC-removed cobalt chloride solution; after resin adsorption is saturated, compressing the residual cobalt chloride solution in the adsorption column to a cobalt chloride stock solution tank, and washing the adsorption column with water; after water washing is finished, steam is introduced to blow off the resin adsorption column, organic matter enriched in the resin is analyzed and separated, and obtained desorption liquid is fed into a desorption liquid collecting tank; after the air stripping is completed, cooling the resin in the adsorption column by using cold water until a preset temperature is reached; and putting the desorbed resin adsorption column into the next adsorption process for recycling. According to the method, the oil removal rate and the oil removal stability can be improved, recycling of the oil removal material is achieved, the technological process is simple, the automation degree is high, dangerous solid waste cannot be generated, and the operation cost is reduced.
Owner:GEM JIANGSU COBALT IND CO LTD
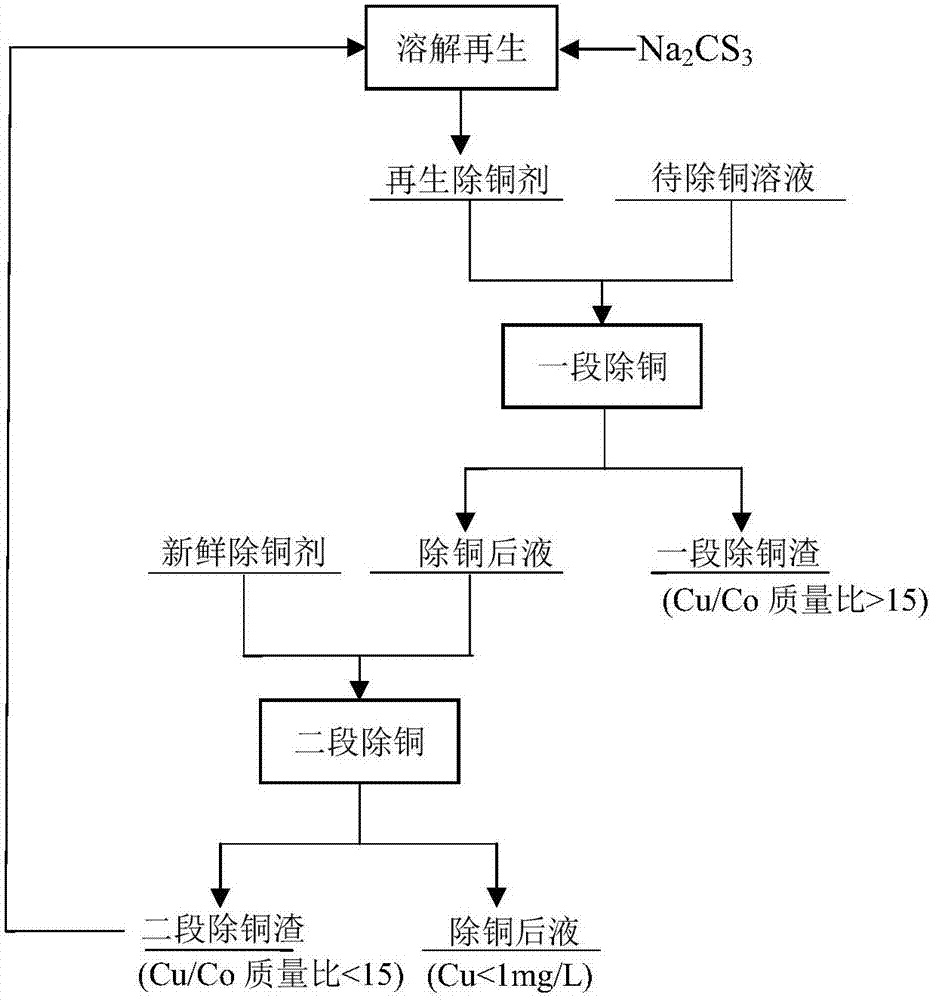
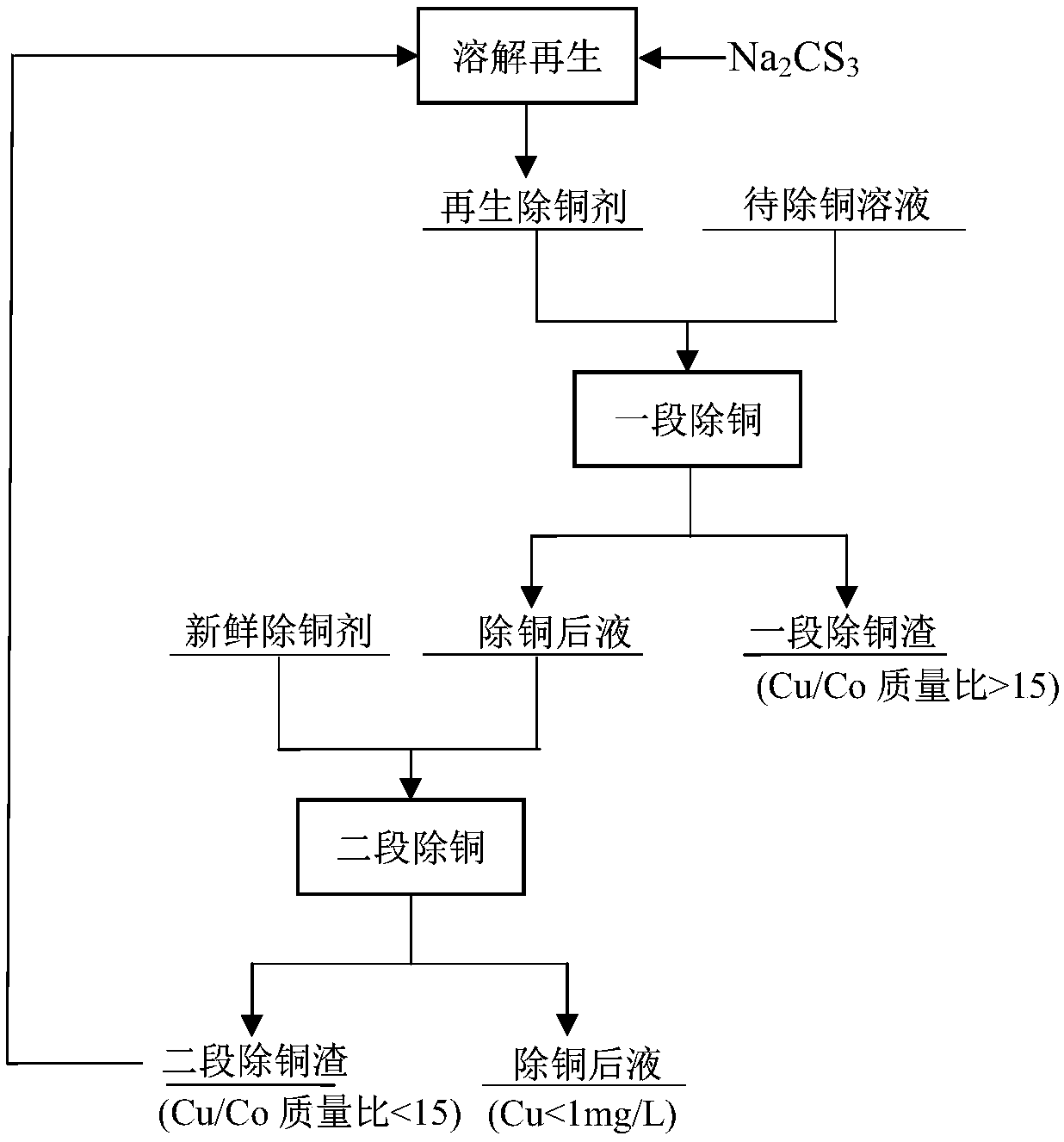
Method for deeply removing copper from cobalt salt solution
The invention relates to a method for deeply removing copper from a cobalt salt solution. A two-stage copper removal technology is adopted for removing copper from a cobalt chloride solution. A regenerated copper removal agent used in a first stage of copper removal is sourced from copper removal slag produced by a second stage of the copper removal, and finally qualified copper slag is discharged; and cobalt thiocarbonate is added in the second stage of the copper removal, so that a qualified copper-removed liquid is produced. The method provided by the invention has the advantages that the characteristic that the copper removal slag can be regenerated into the copper removal agent is fully utilized, copper concentration can be reduced to below 1mg / L, and copper removal slag with the copper-cobalt ratio higher than 15 / 1 is obtained.
Owner:CENT SOUTH UNIV
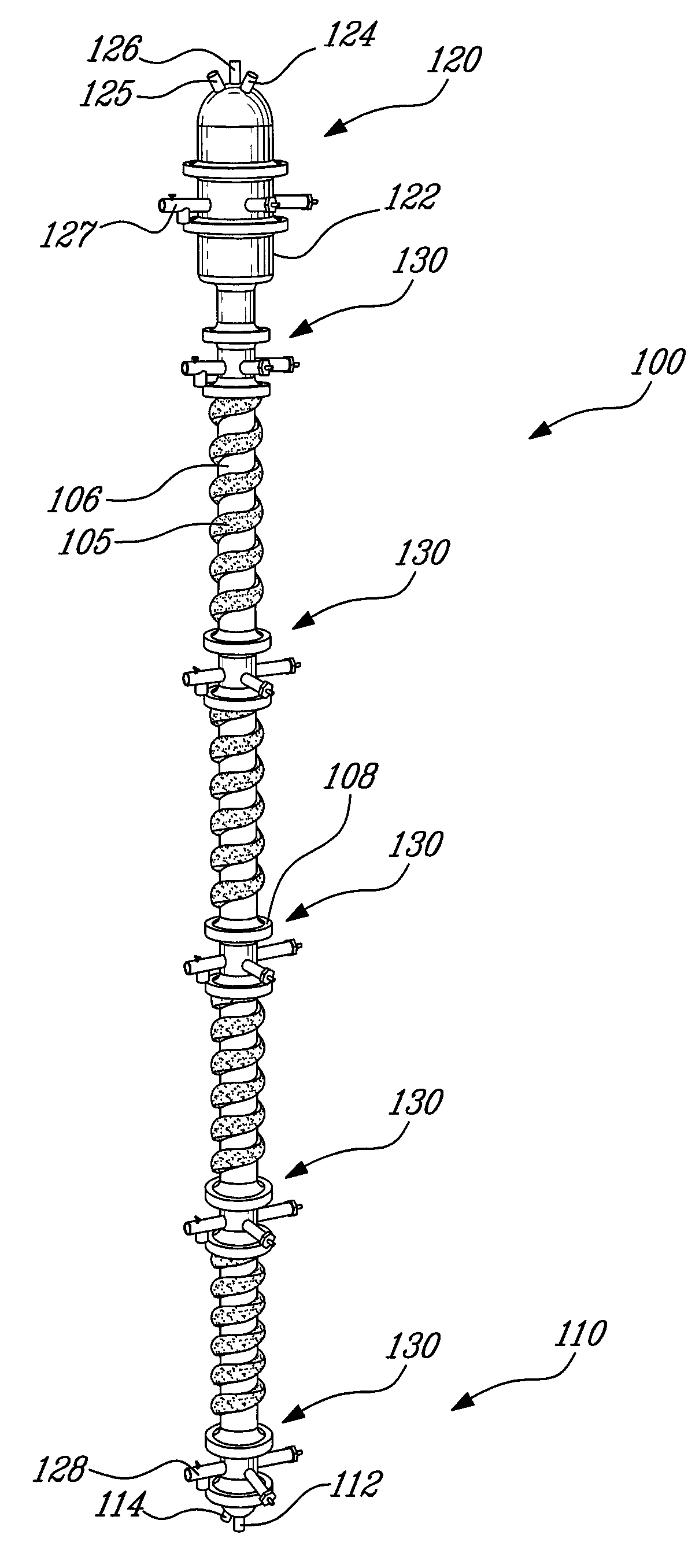
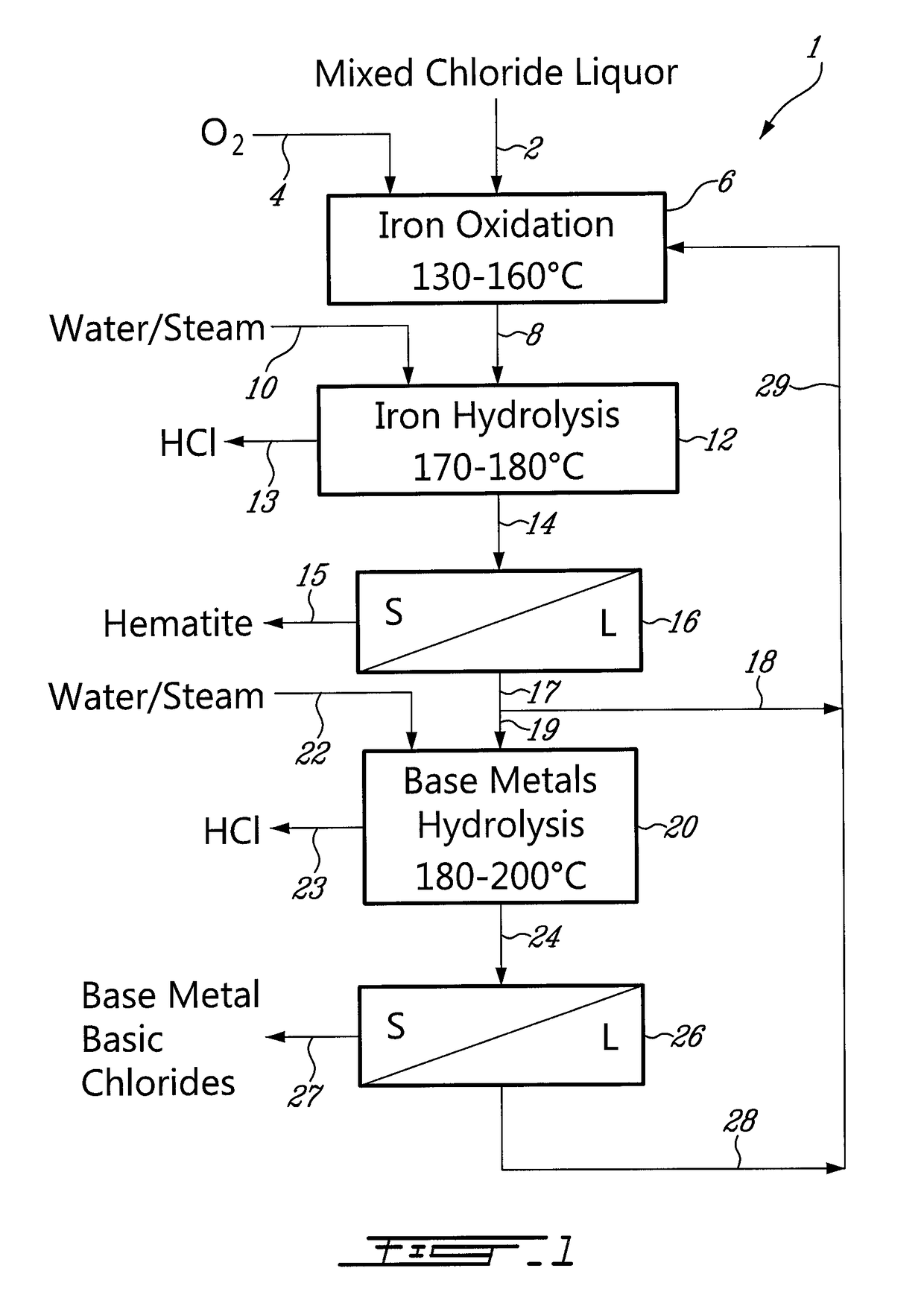
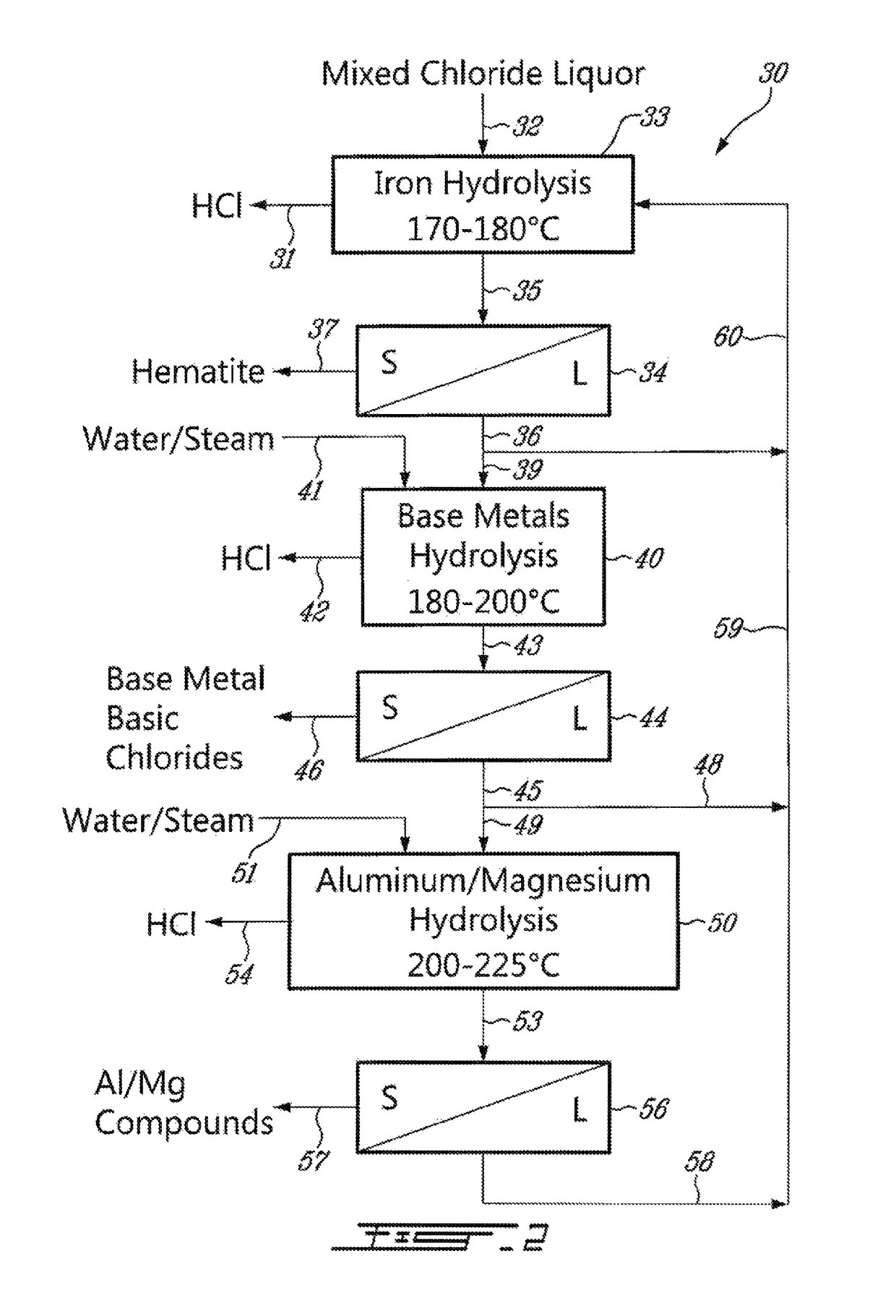
Process for the recovery of metals and hydrochloric acid
A method for recovering hydrochloric acid and metal oxides from a chloride liquor is described. The method uses a chloride liquor including the metal and mixing the liquor and a matrix solution to produce a reaction mixture, wherein the matrix solution assists oxidation / hydrolysis of the metal with HCl production. In a preferred embodiment the matrix solution includes zinc chloride in various stages of hydration and an oxygen containing gas is added to the mix. A method where the improvement is the mixing of a liquor and a matrix solution where the solution assists hydrolysis of the metal with HCl production is also disclosed. The reactor is a column reactor in a preferred embodiment. Further disclosed is the method of using the matrix solution and a reactor for recovering hydrochloric acid and for oxidizing / hydrolysis of a metal.
Owner:NEOMET TECH
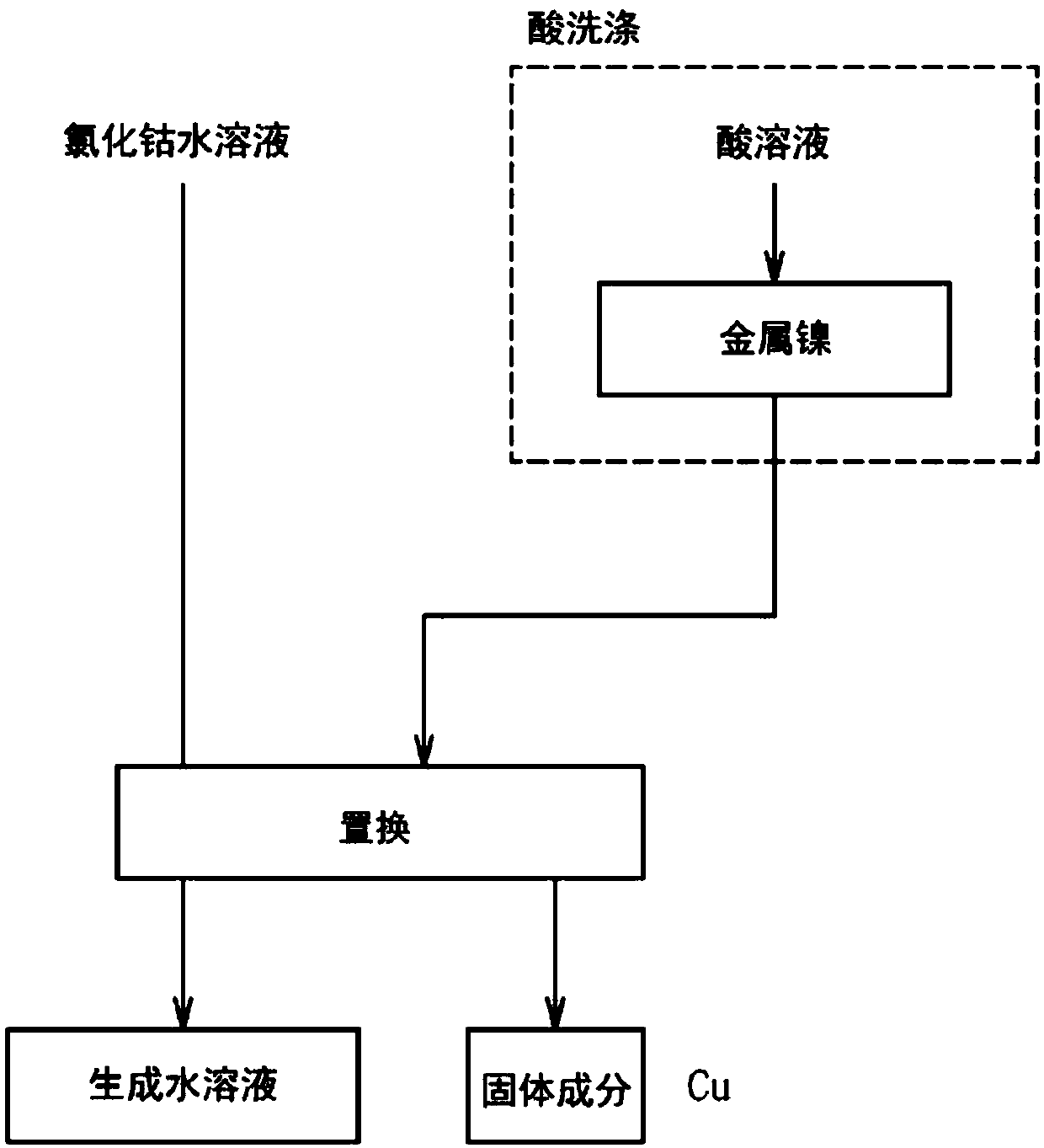
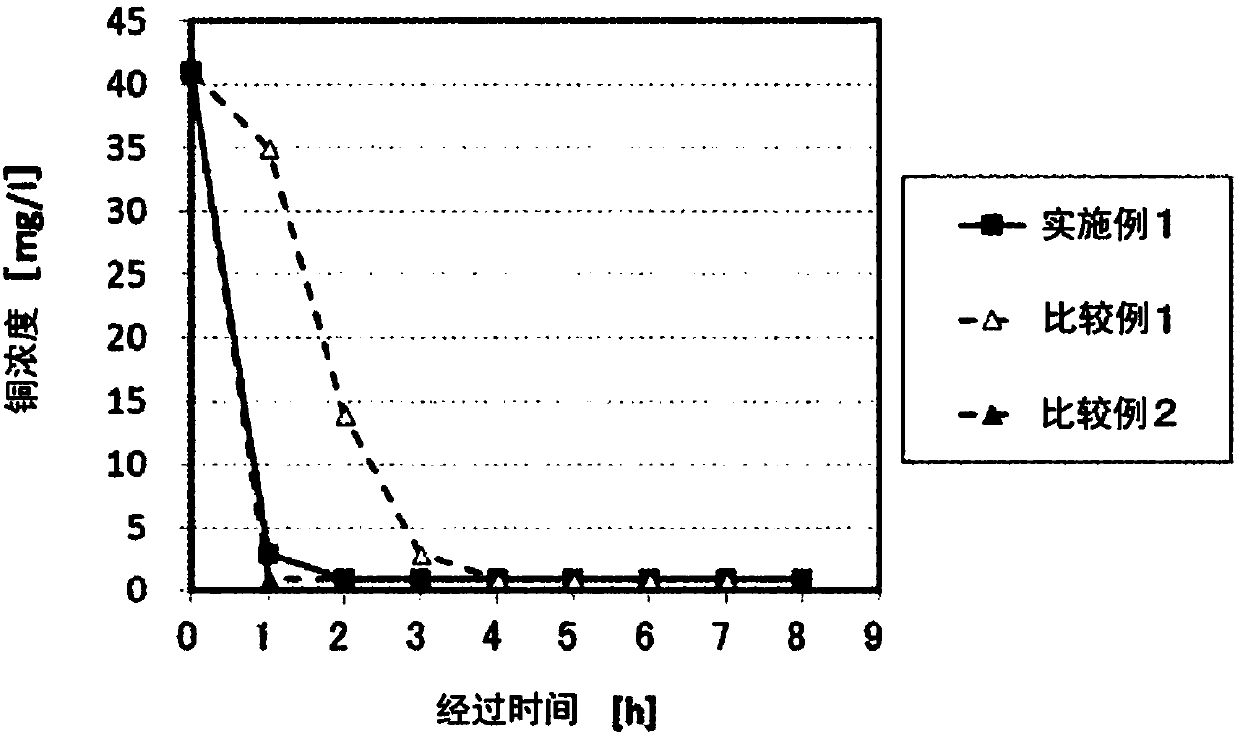
Aqueous cobalt chloride solution purification method
InactiveCN107614711AGood removal effectEasy to operateCobalt halidesCell electrodesPurification methodsAqueous solution
The invention provides an aqueous cobalt chloride solution purification method capable of efficiently removing impurities from a cobalt salt solution. The solution is a method for bringing metallic nickel into contact with an aqueous cobalt chloride-containing solution to remove impurities by a cementation reaction, wherein the metallic nickel is washed with an acidic solution of pH 2.5 or less prior to bringing the metallic nickel into contact with the aqueous cobalt chloride-containing solution. Since the metallic nickel is washed with an acidic solution of pH 2.5 or less, the passive film on the surface of the metallic nickel is removed. Since the passive film has been removed, when the metallic nickel is brought into contact with the aqueous cobalt chloride-containing solution, impurities that are more electropositive than metallic nickel can be precipitated by a cementation reaction. Moreover, since the metallic nickel need only be washed with acid and brought into contact with the aqueous cobalt chloride-containing solution, it is possible to remove impurities from the aqueous cobalt chloride-containing solution easily.
Owner:SUMITOMO METAL MINING CO LTD
A method for deep copper removal in cobalt salt solution
Owner:CENT SOUTH UNIV
Gel, method of forming the same, photovoltaic device and method of forming the same
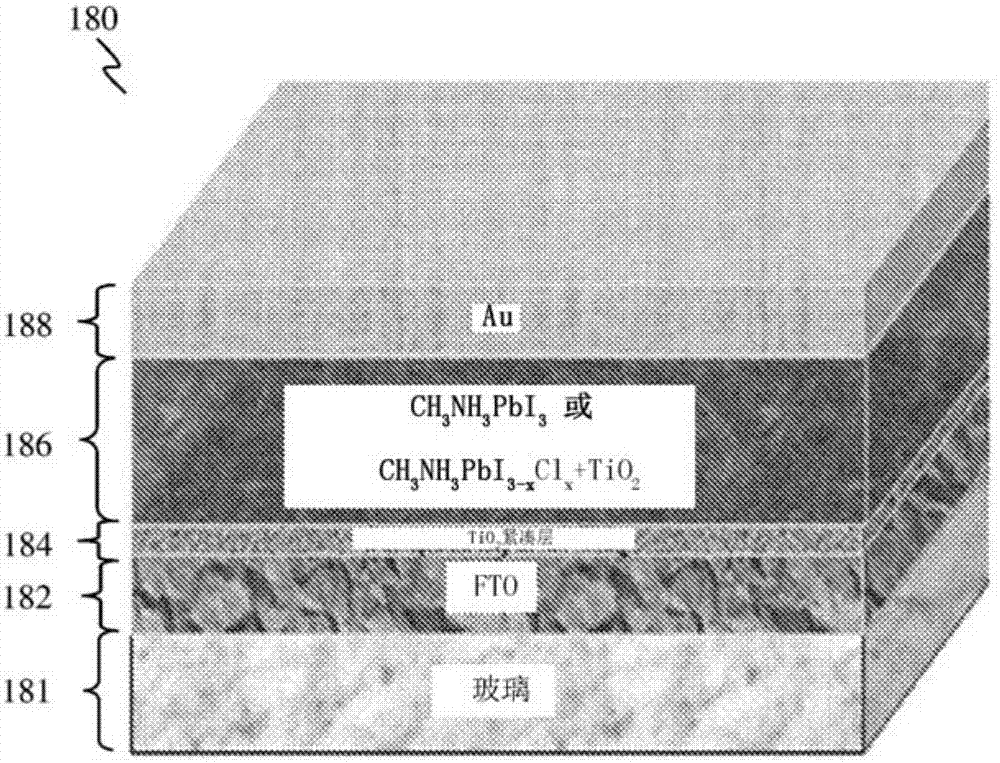
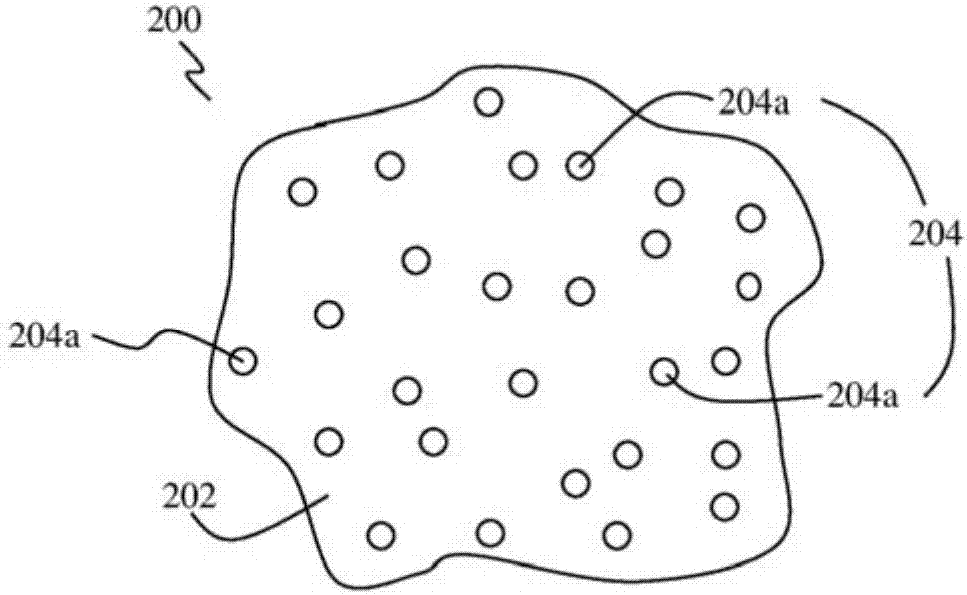
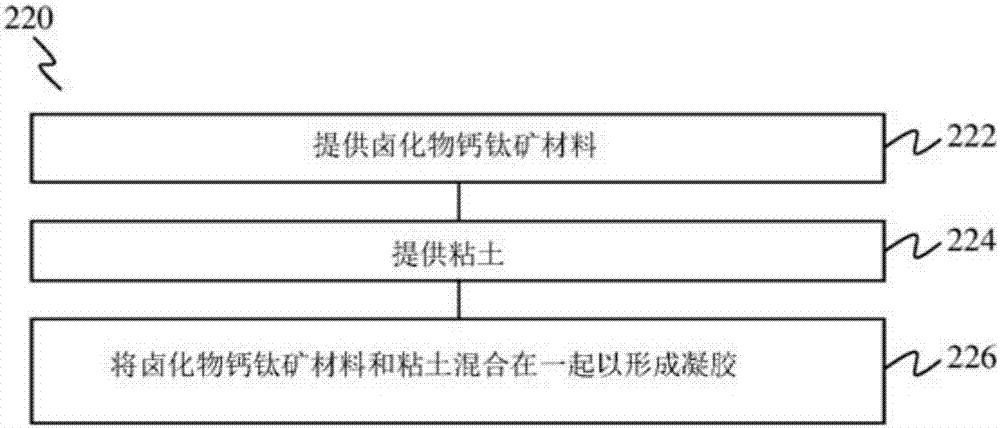
According to the embodiments of the present invention, a gel is provided. The gel has a composition including clay nanoparticles and a halide perovskite material of the general formula AMX3, where A may be an organic or inorganic ion, M may be a metal cation or element, and X may be a halogen anion or element, such as methylammonium lead iodide (CH3NH3Pbl2). According to further embodiments of thepresent invention, a method of forming a gel, a photovoltaic device and a method of forming a photovoltaic device are also provided.
Owner:NANYANG TECH UNIV +1
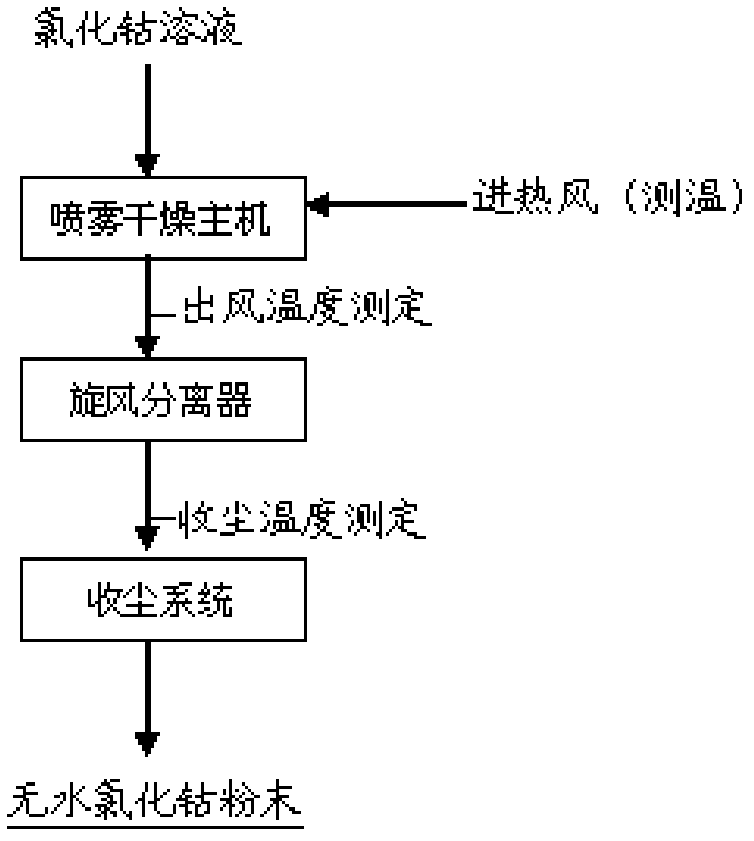
Method for directly preparing anhydrous cobalt chloride powder from cobalt chloride solution
ActiveCN102357308BUniform particle sizeSimple and fast operationCobalt halidesEvaporation by sprayingCycloneVolumetric Mass Density
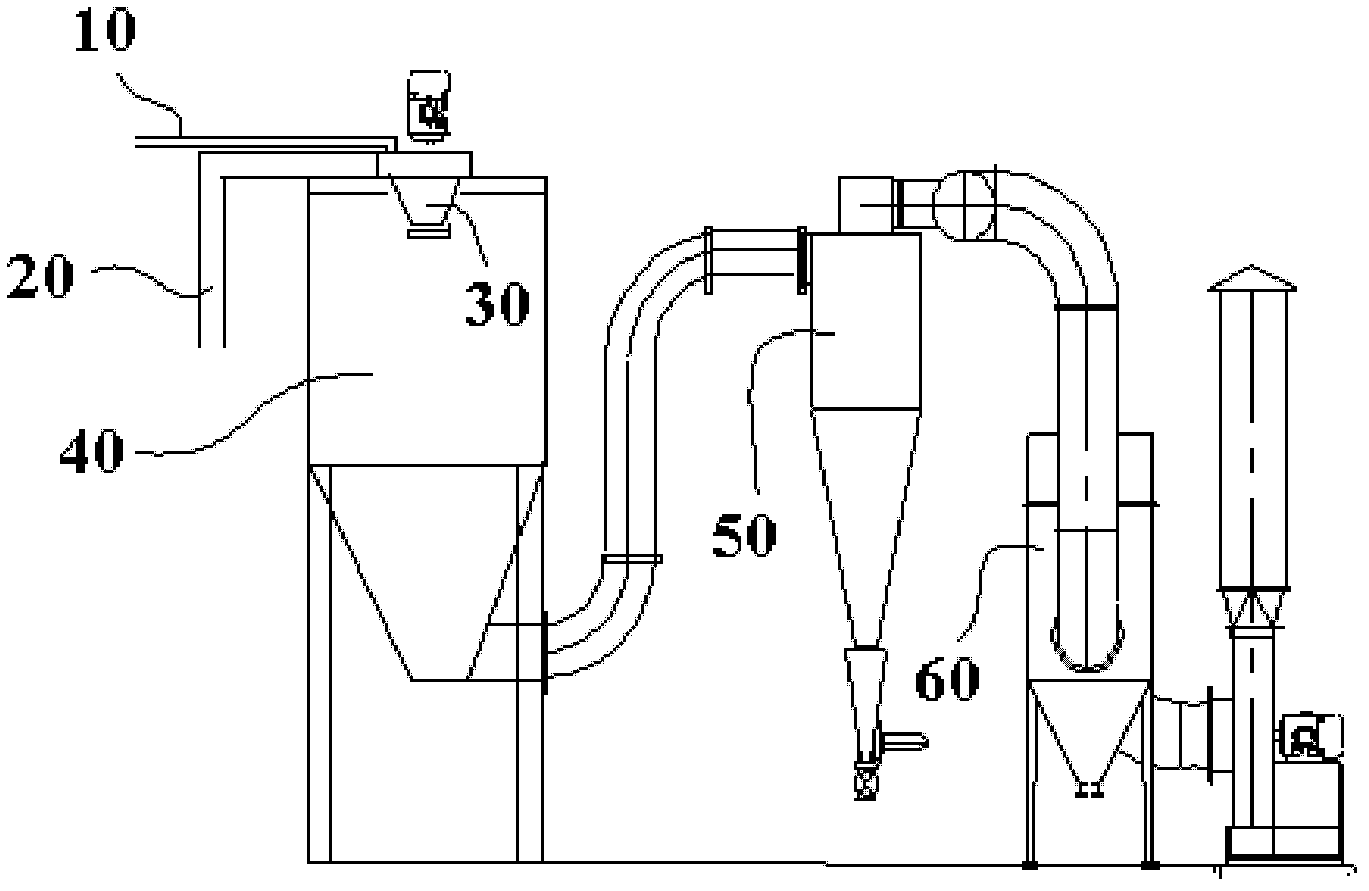
A method for directly preparing anhydrous cobalt chloride powder from cobalt chloride solution is characterized by heating air through an air heater to convert the air into hot air, enabling the hot air to enter an atomizing drying chamber in spiral mode under the condition that air inlet temperature of a cyclone in the drying chamber is controlled between 230 DEG C to 280 DEG C, and air outlet temperature of the cyclone in the drying chamber is controlled between 130 DEG C to 200 DEG C, and simultaneously locating the cobalt chloride solution with the cobalt density ranging from 80g / L to 130g / L into a centrifugal atomizer at the top of the atomizing drying chamber in pumping mode to enable the cobalt chloride solution to be atomized into liquid mist drops and contacted with the hot air in parallel flowing mode, so that water in the cobalt chloride solution can be quickly evaporated, and the cobalt chloride solution can be instantaneously dried to be the anhydrous cobalt chloride powder. Atomizing drying equipment is matched with an auxiliary dust collection system to collect product, the atomizing drying chamber, the cyclone separator and the auxiliary dust collection system can be made of corrosion resistant materials. The anhydrous cobalt chloride powder can be collected through the dust collection system made of corrosion resistant materials after being prepared by atomizing and drying the cobalt chloride solution. The method is convenient to operate, large in output, simple in process and capable of both improving product collection rate and reducing production cost.
Owner:江西江钨钴业有限公司
Crystallization method for cobalt chloride solution
The invention discloses a crystallization method for a cobalt chloride solution. The method comprises the following steps: evaporating and concentrating the cobalt chloride solution; cooling the evaporated and concentrated cobalt chloride solution in a water bath kettle to enable cobalt chloride crystal grains to naturally grow, and turning off the water bath kettle when the temperature of the water bath kettle is lowered to 30 to 35 DEG C; performing natural grain growth on the naturally growing cobalt chloride crystal grains at room temperature for 15 to 20h, and performing suction filtration to obtain a cobalt chloride crude product; performing liquid-solid separation when the temperature of the cobalt chloride crude product is lowered to 20 to 25 DEG C, naturally air-drying the cobaltchloride crude product subjected to liquid-solid separation to obtain a refined cobalt chloride product of which the particle sizes are relatively large and the quality accords to a national standard.The crystallization rate of the obtained cobalt chloride product can reach 60 to 75 percent and, in addition, the crystal grains are uniform in size and crystal color.
Owner:JINCHUAN GROUP LIMITED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com