Aqueous cobalt chloride solution purification method
A purification method and an aqueous solution technology, which are applied in chemical instruments and methods, cobalt halides, cobalt compounds, etc., can solve problems such as poor separation of copper, and achieve the effect of reducing the concentration of impurities
Inactive Publication Date: 2018-01-19
SUMITOMO METAL MINING CO LTD
View PDF5 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
On the other hand, since the solubility product of copper is 2.2×10 -20 , the solubility of copper at pH6 is 14mg-copper / L, so the separation of copper becomes worse
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
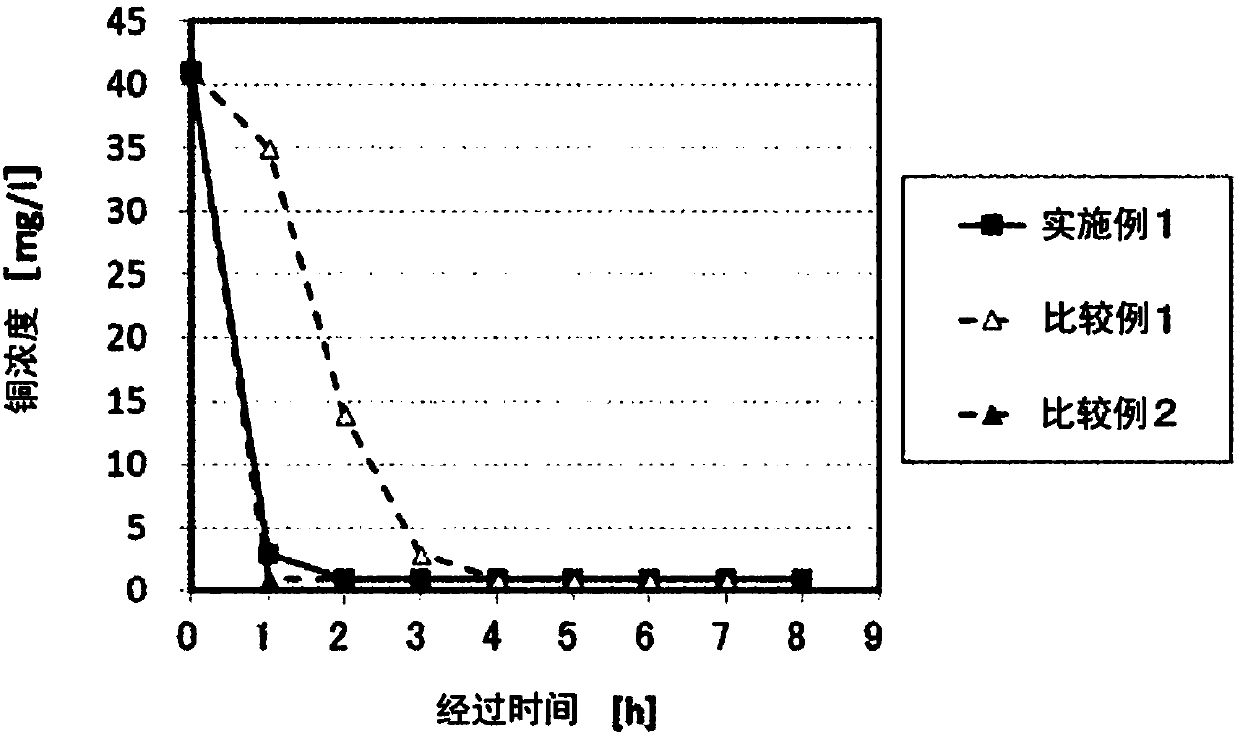
Experimental program
Comparison scheme
Effect test
Embodiment 1
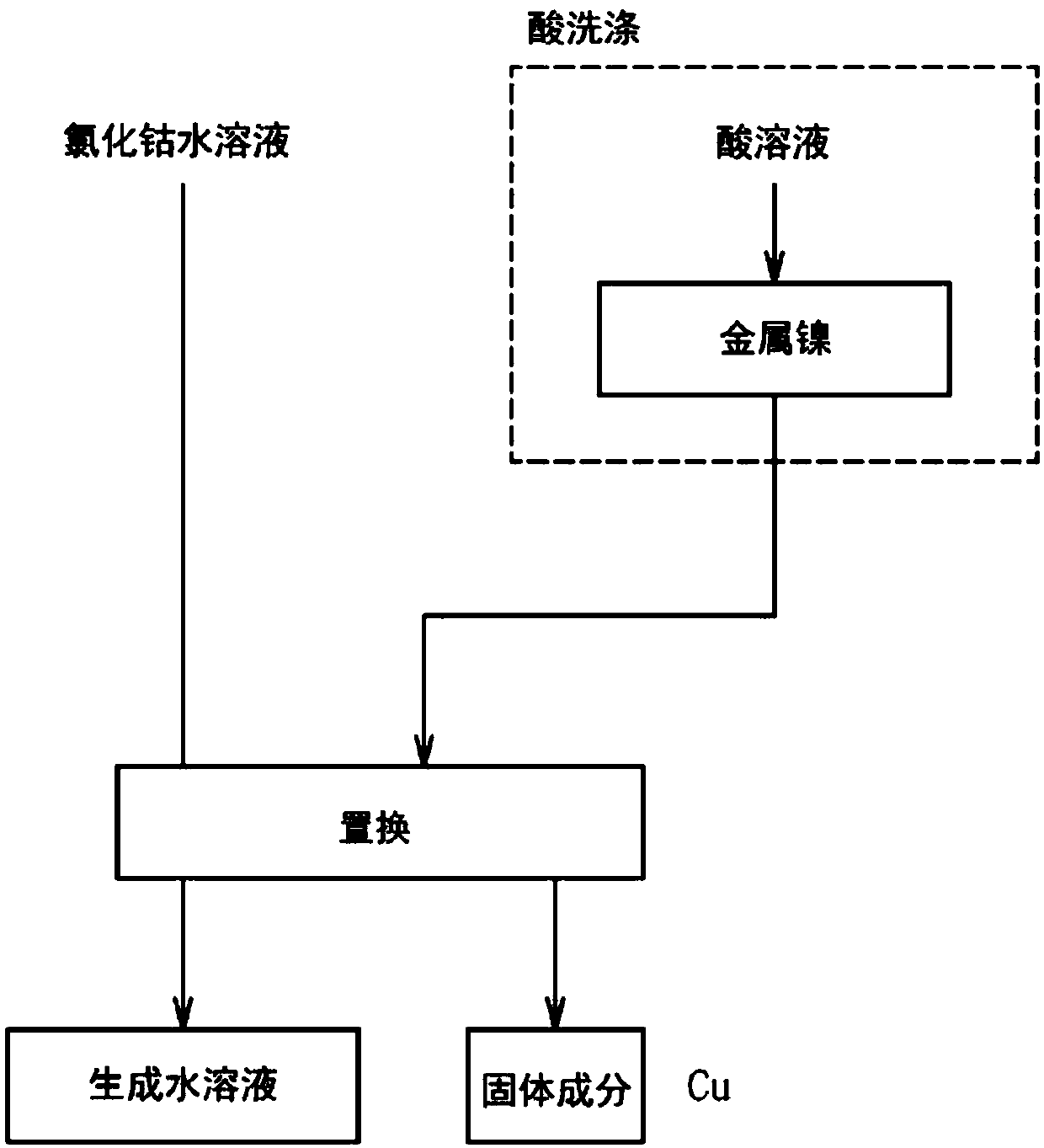
[0073] 40 g of pulverized nickel agglomerates were immersed in 40 ml of 3 mol / L hydrochloric acid for 5 minutes, and washed with acid (pickling treatment).
[0074] The pulverized material of the nickel agglomerate was added to an aqueous cobalt chloride solution at room temperature (20° C.), and stirred and mixed for 8 hours.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility coefficient | aaaaa | aaaaa |
Login to View More
Abstract
The invention provides an aqueous cobalt chloride solution purification method capable of efficiently removing impurities from a cobalt salt solution. The solution is a method for bringing metallic nickel into contact with an aqueous cobalt chloride-containing solution to remove impurities by a cementation reaction, wherein the metallic nickel is washed with an acidic solution of pH 2.5 or less prior to bringing the metallic nickel into contact with the aqueous cobalt chloride-containing solution. Since the metallic nickel is washed with an acidic solution of pH 2.5 or less, the passive film on the surface of the metallic nickel is removed. Since the passive film has been removed, when the metallic nickel is brought into contact with the aqueous cobalt chloride-containing solution, impurities that are more electropositive than metallic nickel can be precipitated by a cementation reaction. Moreover, since the metallic nickel need only be washed with acid and brought into contact with the aqueous cobalt chloride-containing solution, it is possible to remove impurities from the aqueous cobalt chloride-containing solution easily.
Description
technical field [0001] The present invention relates to the purification method of cobalt chloride aqueous solution. Background technique [0002] Cobalt is a rare metal and is a precious metal used as a material of an alloy. In addition, cobalt is also used as an electrode material for batteries for purposes other than alloys. For example, cobalt is also used as a positive electrode material of a lithium ion battery which is a non-aqueous electrolyte secondary battery for vehicles developed in recent years. [0003] When manufacturing a positive electrode material for a lithium ion battery as such a nonaqueous electrolyte secondary battery, a metal hydroxide called a precursor is generally formed by neutralizing an aqueous solution of a metal salt mixed at a prescribed ratio of. When this precursor is mixed with a lithium compound and fired, a positive electrode material can be produced. Furthermore, when producing a positive electrode material containing cobalt, a salt...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C22B23/06C01G51/08C22B3/46C22B23/00H01M4/525
CPCC22B3/46H01M4/525C22B23/0469C01G51/08Y02P10/20C01G51/085C22B23/06
Inventor 大原秀树浅野聪高野雅俊丹敏郎
Owner SUMITOMO METAL MINING CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com