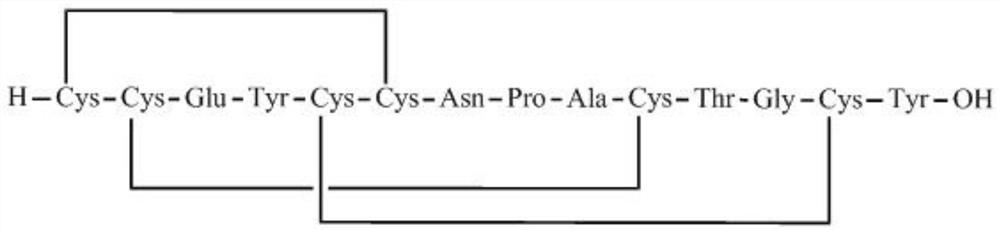
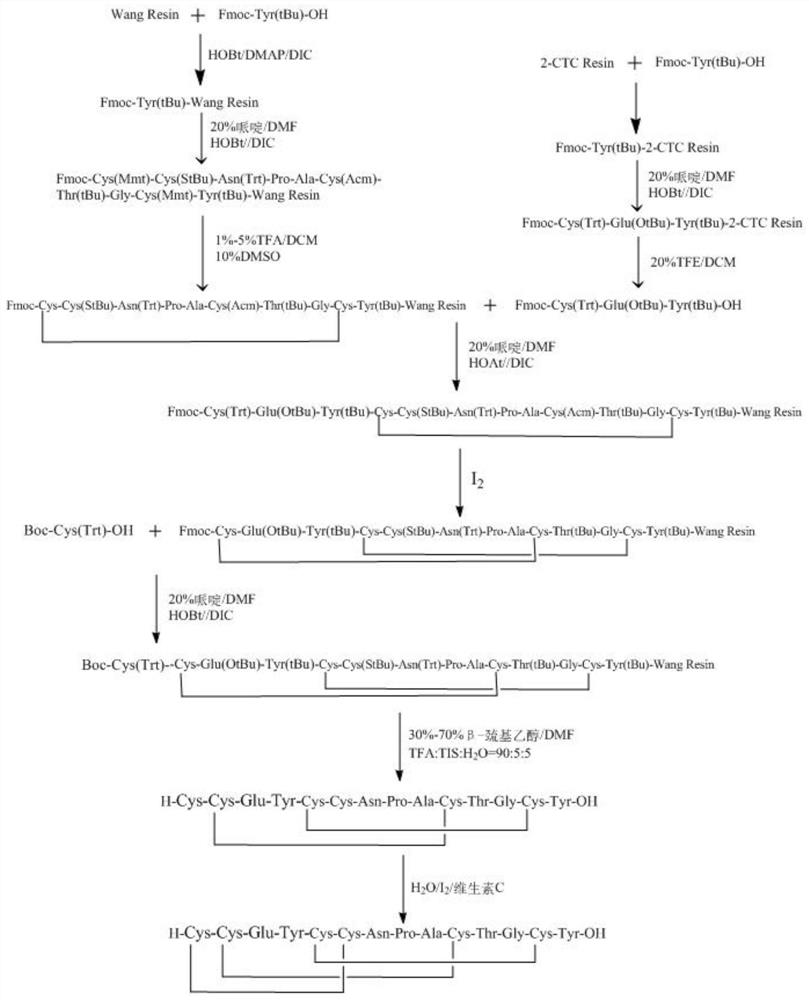
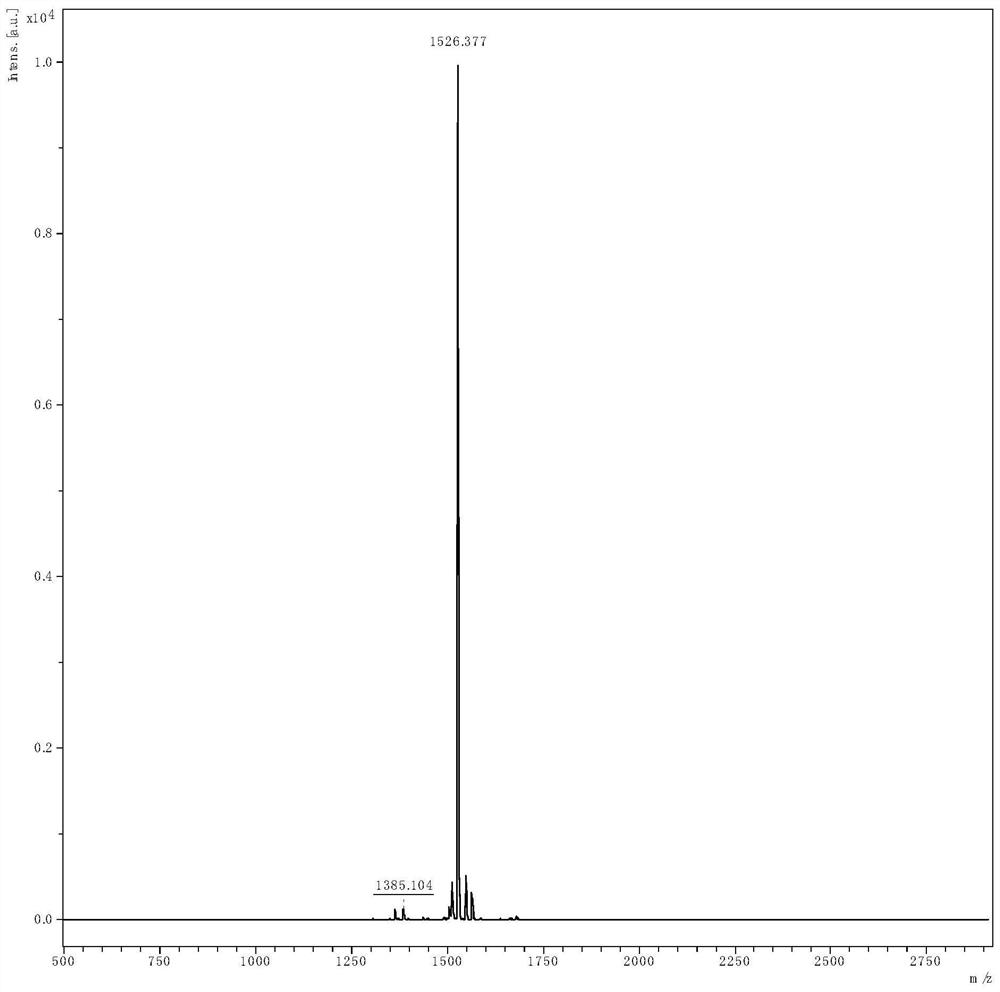
Preparation method of linaclotide
A technology of linaclotide and linaclotide is applied in the field of preparation of linaclotide, which can solve the problems of low purity and yield of linaclotide, unfavorable purification and separation of products, unfavorable amplification and production, etc. Low price, easy to scale up production, reduce pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The present invention discloses a preparation method of linaclotide, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0035] according to figure 2 According to the route shown, the crude linaclotide is synthesized, dissolved and filtered, and then purified by HPLC to obtain linaclotide refined peptide. Its st...
Embodiment 1
[0052] Embodiment 1: the synthesis of Fmoc-Tyr (tBu)-Wang Resin
[0053] Weigh 6.713 grams of Wang Resin with a substitution degree of 0.81 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes. Weigh 6.893 grams of Fmoc-Tyr(tBu)-OH, 2.432 grams of HoBT, 0.220 grams of DMAP, dissolve with 30ml of DMF, add 3.018 grams of DIC to activate for 5min, add the mixed solution to the reaction column, and react at room temperature for 2-3 hours, the reaction process Nitrogen was used as protective gas. After the reaction was finished, the reaction liquid was drawn off, the resin was washed 3 times with DMF, and acetic anhydride and pyridine were added according to the molar ratio (1:1) to carry out the blocking reaction for 6 hours. The resin was shrunk with methanol and dried in vacuum to obtain Fmoc-Tyr(tBu)-Wang Resin. The degree of substitution of Fmoc-Tyr(tBu)-Wang Resin measured by ultraviolet spectrophotometer was 0...
Embodiment 2
[0054] Example 2: Synthesis of Linaclotide (5-14)-Wang Resin
[0055] Weigh 6.383 grams of Fmoc-Tyr(tBu)-Wang Resin with a degree of substitution of 0.47mmol / g, transfer it to a solid-phase reaction column, wash it twice with DMF, swell the resin with DMF for 30 minutes, and add 20% piperidine / DMF (V / V) solution for 5+10 minutes to remove the Fmoc protecting group on the peptide resin, after removal, wash the resin with DMF for 6 times, and the ninhydrin test result was positive, indicating that the deprotection was successful. Weigh 5.542 grams of Fmoc-Cys(Mmt)-OH, 1.459 grams of HOBt, dissolve with 15ml of DMF, add 1.688ml of DIC to activate for 5 minutes under ice-water bath conditions, add the mixed solution to the reaction column, react at room temperature for 2 hours, and react Nitrogen was used as a protective gas during the process, and the ninhydrin detection resin was colorless and transparent, indicating that the condensation was successful.
[0056] After the rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com