Application of musclin in preparation of drugs for inhibiting muscle adiposis, fibrosis and atrophy
A technology for muscle fat and fibrosis, applied in drug combinations, muscular system diseases, neuromuscular system diseases, etc., can solve the problems that the medicinal value of Musclin has not been fully developed, Musclin muscle cells are not mentioned, and Musclin's application range is limited.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Research on the effect of Musclin on inhibiting muscle fat formation
[0025] Musclin used in this program was purchased from Shanghai GL Biochem Co., Ltd, sequence: NH 2 - SFSGFGSPLDRLSAGSVEHRGKQRKAVDHSKKR-COOH (SEQ ID NO.1), which is the partial sequence of amino acids 80-112.
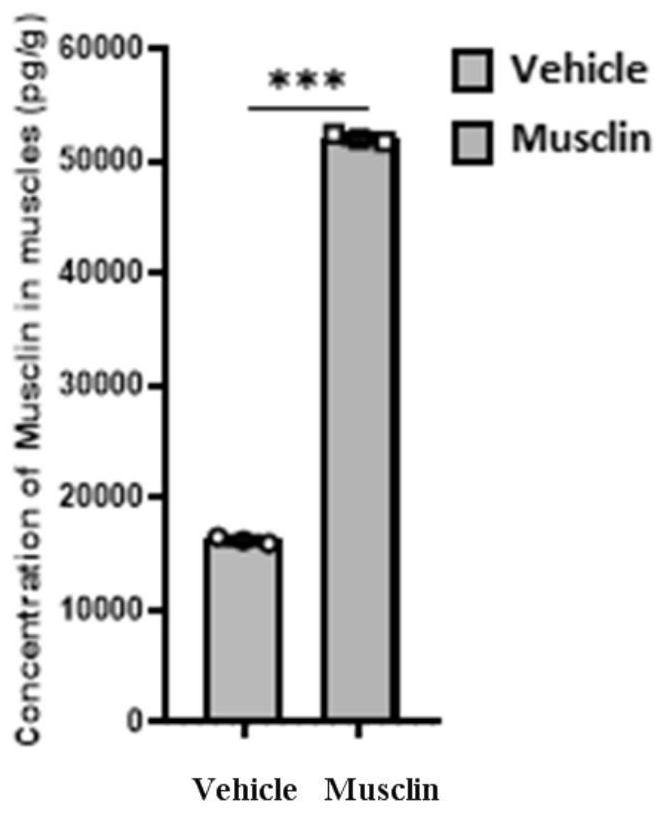
[0026] Musclin overexpression mice were constructed: Musclin recombinant protein was dissolved in 1% BSA solution at 80 μg / mL, Musclin solution was injected continuously for 5 days, once a day, 100 μL each time, and the control group was injected with the same amount of 1% BSA solution. ELISA experiments confirmed that compared with the control group, the Musclin concentration in the mouse muscles was significantly increased (experimental results are as follows: figure 1 As shown, the right is the group injected with Musclin protein, and the left is the group injected with 1% BSA solution), which proves that this method can effectively promote the increase of Musclin protein concen...
Embodiment 2
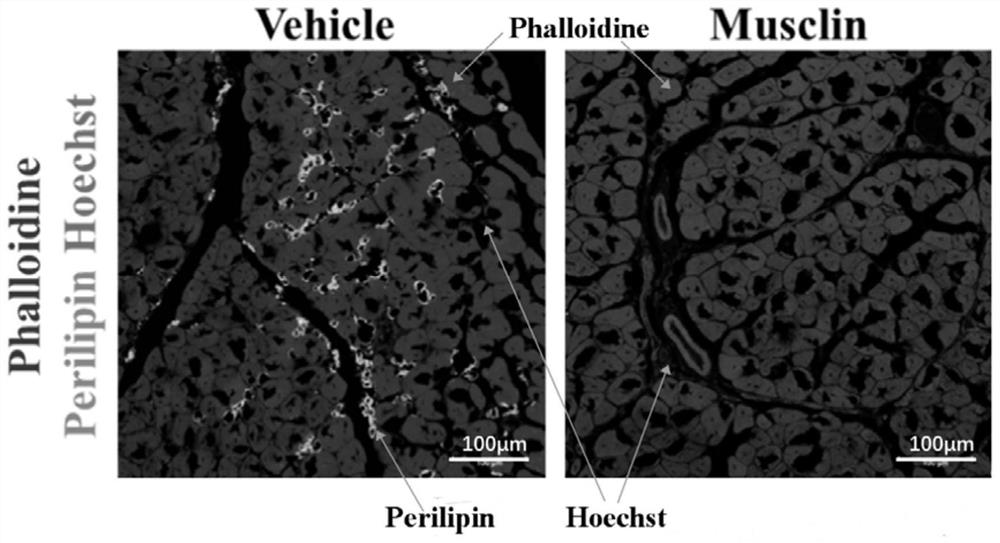
[0031] Example 2: Research on the effect of Musclin on inhibiting muscle fibrosis
[0032] The process of constructing Musclin overexpression mice is the same as that in Example 1.
[0033] Construction of muscle fibrosis mouse model: 50 μL of cardiotoxin (cardiotoxin, CTX) solution (10 μ M) was injected into the tibialis anterior muscle of mice (Musclin overexpression mice or control groups injected with 1% BSA) to induce muscle fibrosis.
[0034] Preparation of specimens: (1) take the tibialis anterior muscle on the 15th day after cardiotoxin injection, fix it in 4% paraformaldehyde for 12 hours, and then dehydrate it overnight with 30% sucrose solution; (2) take the tibialis anterior muscle after dehydration Tissues were prepared into frozen sections with a thickness of 8-10 μm using a cryostat.
[0035] Fluorescent immunohistochemical detection of fibrosis: (1) warm the frozen section to room temperature; (2) rehydrate in PBS; (3) block the sample with blocking solution a...
Embodiment 3
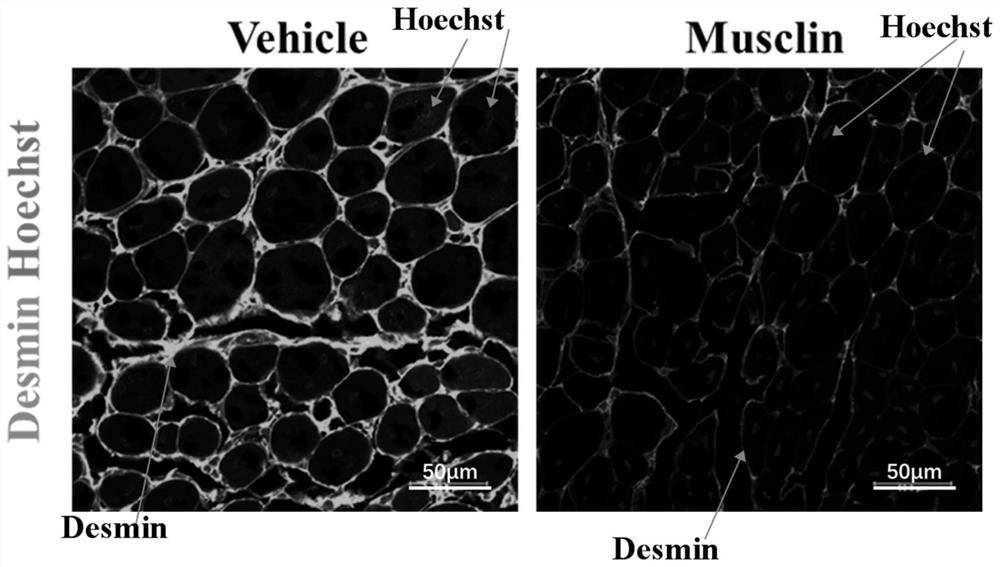
[0037] Embodiment 3: Musclin's research on the effect of treating muscular atrophy
[0038] Construction of muscle atrophy model: After mice were anesthetized with 0.5% pentobarbital sodium, the sciatic nerve and tibialis anterior tendon of one hind limb were cut off, and the sutures were sterilized.
[0039] Musclin treatment: Since the establishment of the model, the Musclin recombinant protein (80 μg / mL) was injected intraperitoneally every other day, and each injection was 100 μL until the time point of sample collection (to 42 days).
[0040] Preparation of specimens: (1) Take the atrophic tibialis anterior muscle at the specified time point, fix it in 4% paraformaldehyde for 12 hours, and then dehydrate it overnight with 30% sucrose solution; (2) press the dehydrated tibialis anterior muscle tissue by 8- Frozen sections were prepared with a thickness of 10 μm using a cryostat.
[0041] Fluorescent immunohistochemical detection of degeneration of atrophic muscle: (1) war...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com