A kind of anti-cancer cachexia compound and its application
A compound and cachexia technology, which is applied in the field of anti-cancer cachexia compounds, can solve the problems of research difficulty, high cost, and clinical treatment difficulties, and achieve the effect of alleviating cancer cachexia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Establishment of a drug screening model targeting the MSTN promoter:
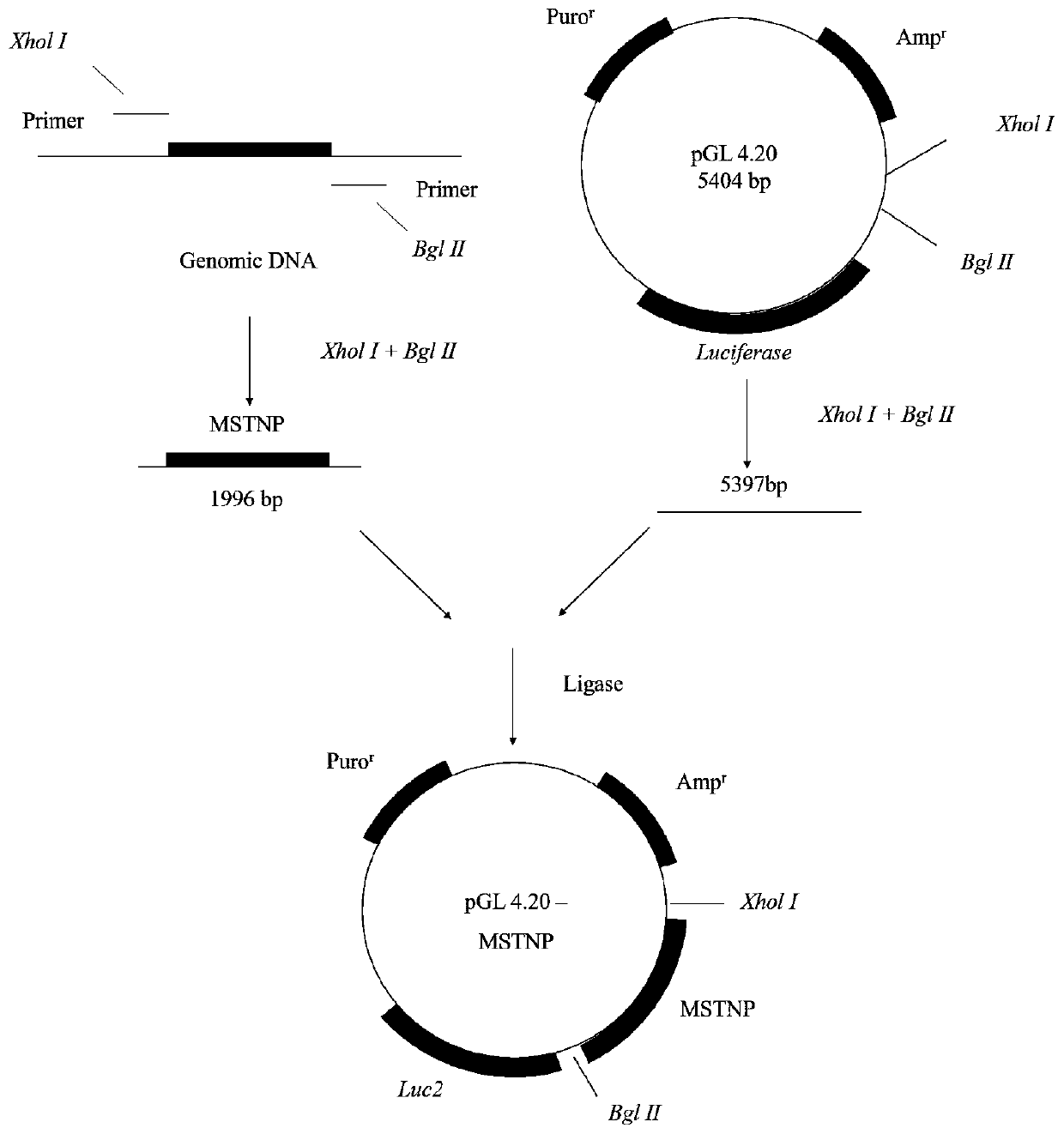
[0063] In the UCSC database, the promoter sequence of mouse MSTN was found, and upstream primers and downstream primers were designed at about -2000bp upstream of the transcription start site and before the downstream translation start site, respectively. Using PCR, enzyme digestion, enzyme linkage, gel recovery and transformation molecular biology techniques, the recombinant plasmid pGL4.20-MSTNP was constructed using the pGL4.20 vector of Promega Company, and the flow chart is as follows figure 1 , wherein, MSTNP is the promoter sequence of MSTN, and pGL4.20 is a plasmid with luciferase (Luciferase) from Promega Company.
[0064] Add restriction sites and protective bases to upstream and downstream primers:
[0065] The sequence of the upstream primer P1 is shown in SEQ ID No.1, and the specific sequence is as follows:
[0066] 5'-CCGCTCGAGAATCCCTTGCCTTCATCTG-3',
[0067] Among them, the underli...
Embodiment 2
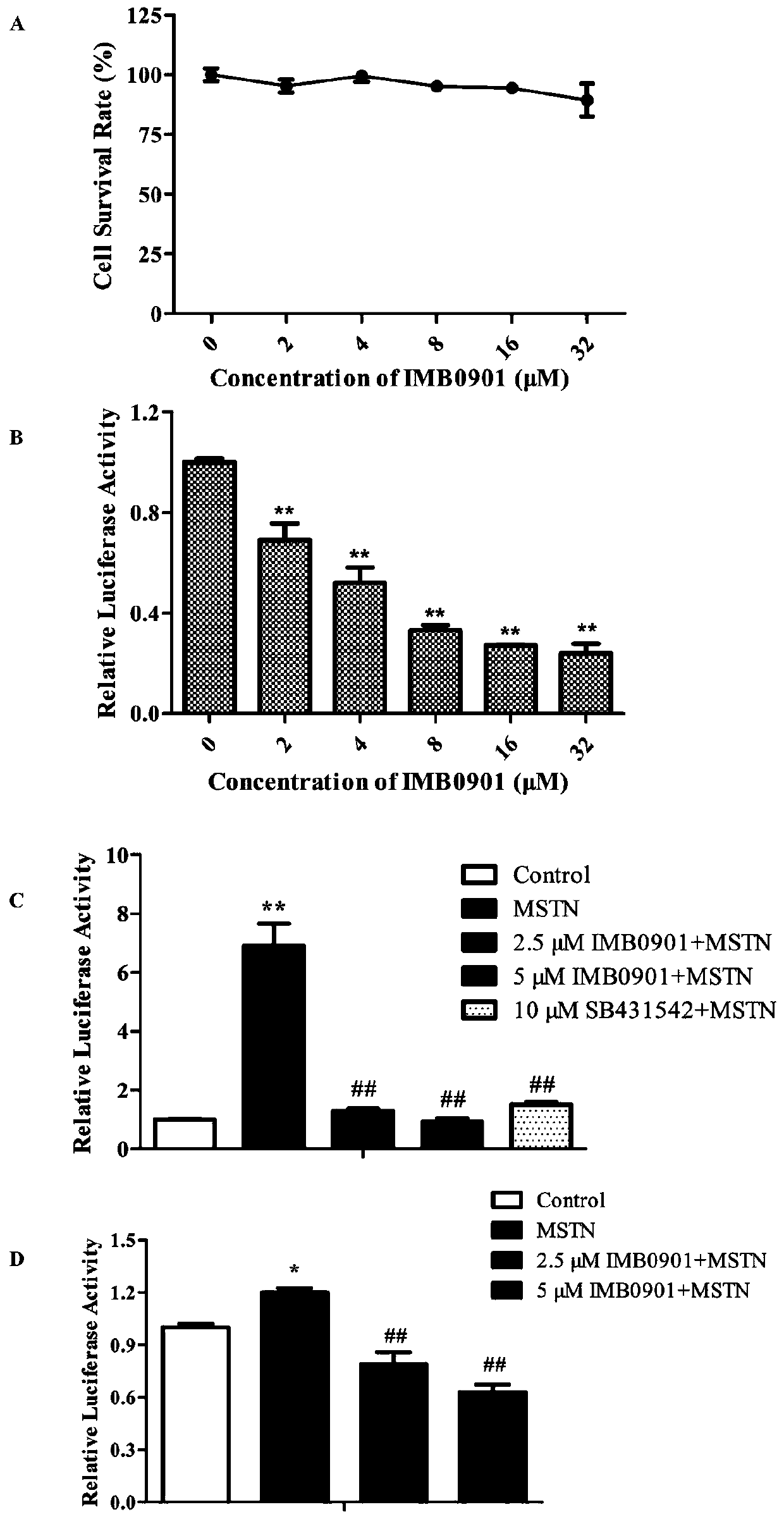
[0083] Inhibitory effect of compounds on MSTN promoter and MSTN signaling pathway:
[0084] The screening process is roughly as follows: Inoculate the HEK293T-MSTNP obtained in Example 1 to 10,000 / well in a polylysine (PDL)-coated 96-well plate, and directly add 0.5 μl of the molecular library after overnight attachment Compound (mother solution concentration 10mg / ml) to a final concentration of 25μg / ml, acted for 24h. Aspirate the supernatant, add 50 μl fresh DMEM medium, and then add 50 μl Bright-Glo TM Reagents (Promega Company), shake the reaction for 5 minutes, transfer 100 μl of the reaction solution to a white plate and read it in a chemiluminescence detector.
[0085] Select a drug with an inhibition rate of more than 70% on the MSTN promoter activity, and perform toxicity exclusion and dose-dependent testing on it, and finally obtain a candidate compound from the 26,000 compounds in the molecular library, and then entrust the American Life Chemicals company to synthe...
Embodiment 3
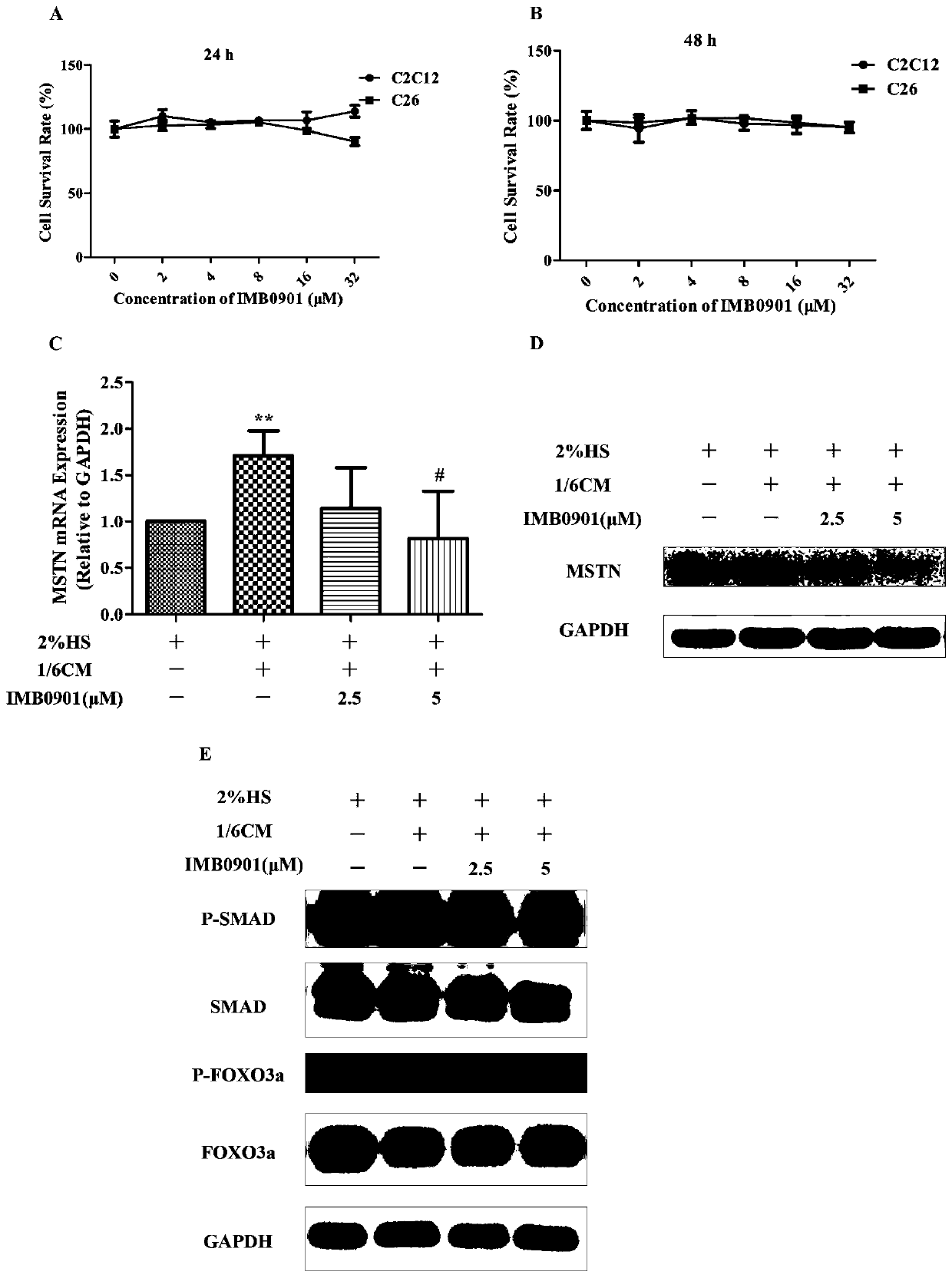
[0089] Western Blot and Real Time PCR were used to detect the effect of compounds on the expression of MSTN and its signaling pathway in the muscle atrophy model in vitro:
[0090] The cytotoxicity of the compounds on C2C12 cells and C26 cells was determined in vitro by the method of MTT. The C2C12 cells and C26 cells in the logarithmic growth phase were digested with trypsin, and the cells were counted. According to the growth rate of the cells, 5000 cells of the C2C12 cells were seeded in a 96-well plate, while the C26 cells were inoculated with PDL. Seed 8000 cells in a 96-well plate, culture at 37°C for 24 hours to allow the cells to adhere to the wall; discard the supernatant medium, and dilute the compound by 2 times with the complete medium, and the concentrations are 2, 4, 8, 16 , 32 μM, add 100 μl to each well, set three duplicate wells for each concentration, set up control group and blank group at the same time, continue to culture at 37°C for 24h and 48h; add 20μl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com