Synthesis method of cyclopropanecarboxylic acid
A synthetic method, the technology of cyclopropanecarboxylic acid, applied in the direction of organic chemistry, etc., can solve the problem of difficult disposal of waste salt, and achieve the effect of less three wastes, easy control, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
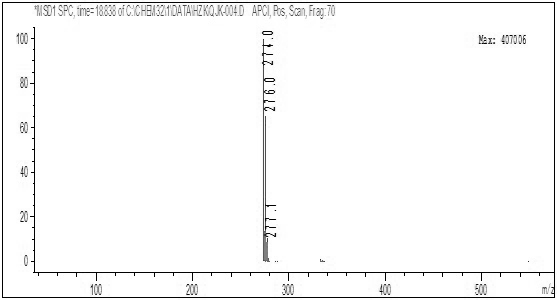
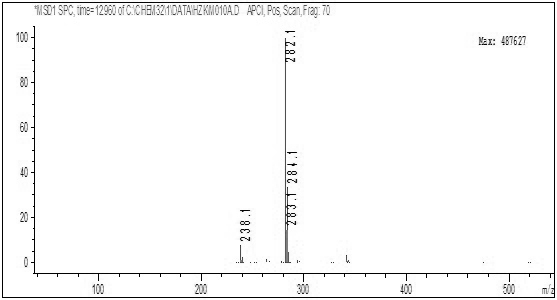
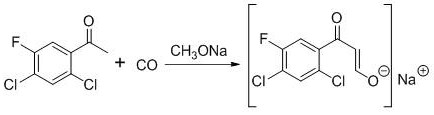
[0053] Add 160.00 g of 2,4-dichloro-5-fluoro-acetophenone, 320.00 g of toluene, and 50.10 g of sodium methoxide (1.2 equivalents) in sequence in a 500 ml reactor, seal the reactor, replace the system with nitrogen three times, and then introduce carbon monoxide until the pressure is 3.8MPa, then raise the temperature to 45°C, at this time, the pressure inside the reactor is 4.5MPa, control the reaction temperature at 50°C, keep the pressure inside the reactor at 4.5MPa, keep the temperature for 5 hours, then the reaction is over, then cool down to 25°C Afterwards, the kettle was opened to obtain the toluene suspension of Intermediate 1. The quality of intermediate 1 in the external standard calculation suspension is 168.84g, and the yield is 85%.
Embodiment 2
[0055] The difference between the method of this example and Example 1 is that the reaction temperature is 55° C., and other steps are the same. The mass of intermediate 1 in the suspension calculated by external standard is 188.70 g, and the yield is 95%.
Embodiment 3
[0057] The difference between the method of this example and Example 1 is that the reaction temperature is 60°C, other steps are the same, the mass of intermediate 1 in the suspension calculated by external standard is 176.78g, and the yield is 89%.
[0058] Embodiment 1 to embodiment 3 experiment summary :
[0059] It can be seen from Examples 1-3 that when the temperature is changed within the range of 45-60°C, the yield of intermediate 1 increases first and then decreases as the temperature increases, so it is determined that the temperature of the present invention is best controlled at 55°C .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com