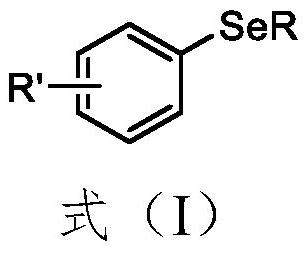
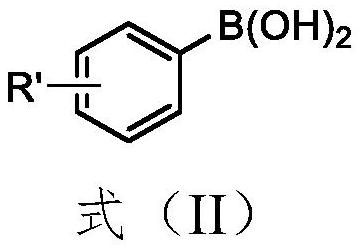
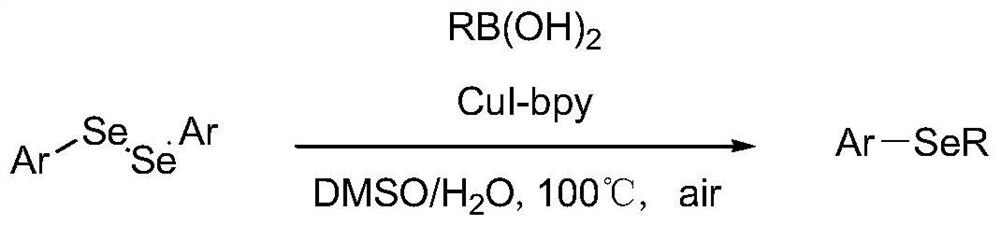Synthesis method of aryl selenide compound
A technology of ether compound and synthesis method, which is applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., can solve the problems of unfavorable industrial expansion, difficult product processing, etc., and achieve low environmental pollution, good tolerance, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Synthesis of p-methoxyphenyl selenide.
[0074] Add 30.4 mg of p-methoxyphenylboronic acid, 23 μL of MeSeSeMe, 66 mg of tetra-n-butylammonium tetrafluoroborate and 10 mL of acetonitrile into a three-port reaction tube equipped with a magnetic stirring bar. In the air, under the condition of 50° C., the electricity was reacted for 3 hours, and the constant current was 6 mA. After the reaction was completed, it was simply filtered, concentrated by rotary evaporation and put on the column. Using pure petroleum ether and 3% ethyl acetate as the mobile phase in turn, 34.8 mg of p-methoxybenzyl selenide was obtained with a yield of 86%.
[0075] Product p-methoxybenzyl selenide: 1 H NMR (400MHz, CDCl 3 ): δ7.41(d, J=8.8Hz, 2H), 6.82(d, J=8.8Hz, 2H), 3.79(s, 3H), 2.30(s, 3H). 13 C NMR (100MHz, CDCl 3 ): δ158.7, 133.4, 121.5, 114.8, 55.3, 8.7.
Embodiment 2
[0076] Example 2 Synthesis of m-methoxybenzyl selenide.
[0077] Add 30.4 mg of m-methoxyphenylboronic acid, 23 μL of MeSeSeMe, 66 mg of tetra-n-butylammonium tetrafluoroborate and 10 mL of acetonitrile into a three-port reaction tube equipped with a magnetic stirring bar. In the presence of air at 50°C, the reaction was carried out with electricity for 3 hours, and the constant current was 6mA. After the reaction was completed, it was simply filtered, concentrated by rotary evaporation, and put on the column. Using pure petroleum ether and 3% ethyl acetate as mobile phases in turn, 24.3 mg of m-methoxybenziselenide was obtained with a yield of 60%.
[0078] The product m-methoxybenzyl selenide: 1 H NMR (400MHz, CDCl 3 ): δ7.18(t, J=8.0Hz, 1H), 7.00(d, J=8.4Hz, 1H), 6.97(s, 1H), 6.74(dd, J=2.4, 2.0Hz, 1H), 3.80 (s,3H),2.36(s,3H). 13 C NMR (100MHz, CDCl 3 ): δ159.8, 133.0, 129.8, 122.4, 115.7, 111.7, 55.2, 7.1.
Embodiment 3
[0079] Example 3 Synthesis of o-methoxybenzyl selenide.
[0080] Add 30.4 mg of o-methoxyphenylboronic acid, 23 μL of MeSeSeMe, 66 mg of tetra-n-butylammonium tetrafluoroborate and 10 mL of acetonitrile into a three-port reaction tube equipped with a magnetic stirring bar. In the presence of air at 50°C, the reaction was carried out with electricity for 3 hours, and the constant current was 6mA. After the reaction was completed, it was simply filtered, concentrated by rotary evaporation and put on the column. Using pure petroleum ether and 5% ethyl acetate as the mobile phase in turn, 27.5 mg of o-methoxybenziselenide was obtained with a yield of 68%.
[0081] The product o-methoxybenzyl selenide: 1 H NMR (400MHz, CDCl 3 ): δ7.24(d, J=7.6Hz, 1H), 7.19(t, J=8.0Hz, 1H), 6.94(t, J=7.6Hz, 1H), 6.82(d, J=8.0Hz, 1H ),3.89(s,3H),2.28(s,3H). 13 CNMR (100MHz, CDCl 3 ): δ128.4, 126.7, 121.5, 121.2, 110.0, 55.8, 4.8.
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com