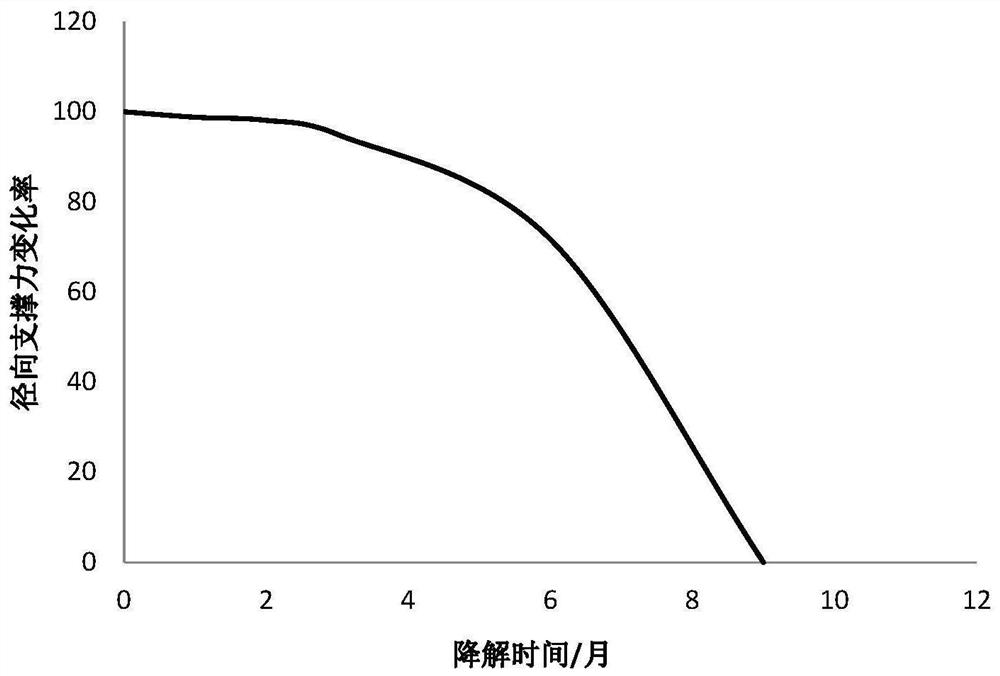
Biodegradable stent and preparation method thereof
A biodegradable polymer technology, applied in stents, medical science, surgery, etc., can solve the problems of vascular stenosis, slow degradation of stent residues, adverse side effects, etc., achieve fast degradation speed, shorten the degradation time of residues, Reduce the effect of adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A degradable stent for lower extremity arteries was prepared, with a size specification of 2.0 mm.
[0034] The preparation method is as follows:
[0035] S1. Dry two kinds of lactic acid pellets with a molecular weight of 700,000 and a molecular weight of 400,000 as raw materials. The drying temperature of the polylactic acid pellets with a molecular weight of 700,000 is 120°C, and the drying time is 4 hours; polylactic acid with a molecular weight of 400,000 The drying temperature of the pellets is 100°C, and the drying time is 4 hours;
[0036] S2. Put the dried 700,000 molecular weight polylactic acid pellets into the first extrusion chamber and the third extrusion chamber of the three-screw extruder, and put the dried 400,000 molecular weight polylactic acid pellets into the three-screw extruder The second extrusion bin of the machine; the extrusion process parameters are: the temperature of the screw zone of the first extrusion bin and the third extrusion bin is ...
Embodiment 2
[0040] A degradable stent for lower extremity arteries was prepared, with a size of 4.0 mm.
[0041] The preparation method is as follows:
[0042] S1. Dry two kinds of lactic acid pellets with a molecular weight of 700,000 and a molecular weight of 400,000 as raw materials. The drying temperature of the polylactic acid pellets with a molecular weight of 700,000 is 120°C, and the drying time is 4 hours; polylactic acid with a molecular weight of 400,000 The drying temperature of the pellets is 100°C, and the drying time is 4 hours;
[0043]S2. Put the dried 700,000 molecular weight polylactic acid pellets into the first extrusion chamber and the third extrusion chamber of the three-screw extruder, and put the dried 400,000 molecular weight polylactic acid pellets into the three-screw extruder The second extrusion bin of the machine; the extrusion process parameters are: the temperature of the screw zone of the first extrusion bin and the third extrusion bin is 218 °C, the tem...
Embodiment 3
[0047] A coronary degradable stent was prepared, with a size specification of 3.0 mm.
[0048] The preparation method is as follows:
[0049] S1. Dry two kinds of lactic acid pellets with a molecular weight of 800,000 and a molecular weight of 300,000 as raw materials. The drying temperature of the polylactic acid pellets with a molecular weight of 800,000 is 123°C, and the drying time is 4 hours; polylactic acid with a molecular weight of 400,000 The drying temperature of the pellets is 95°C, and the drying time is 4 hours;
[0050] S2. Put the dried 800,000 molecular weight polylactic acid pellets into the first extrusion chamber and the third extrusion chamber of the three-screw extruder, and put the dried 300,000 molecular weight polylactic acid pellets into the three-screw extruder The second extrusion bin of the machine; the extrusion process parameters are: the temperature of the screw zone of the first extrusion bin and the third extrusion bin is 220°C, the temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com