Application of Nickel Metal Organic Framework Catalyst in Dimerization of Ethylene to Produce 1-Butene
A technology of metal-organic frameworks and catalysts, applied in the direction of organic compound/hydride/coordination complex catalysts, organic chemistry, physical/chemical process catalysts, etc., can solve the problem of high pressure requirements for ethylene, and achieve cheap raw materials and advanced technology Simple, easy-to-composite effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11D
[0044] Example 1 Synthesis and use of 1D Ni-MIL-77 catalyst
[0045] (1) The synthesis method of 1D Ni-MIL-77 material is as follows: 0.004mol Ni(NO 3 ) 2 ·6H 2 O, 0.006mol glutaric acid and 0.008mol KOH were dissolved in 44ml of mixed solvent with a volume ratio of ethanol and water of 1:1. After fully stirring, the mixed solution was dispersed in 0.4mol / L lye solution, and the stirring was continued for 30min. Transfer to a hydrothermal kettle, react at 180°C and autogenous pressure for 48 hours, then take out the reactor and cool to room temperature naturally, the obtained product is collected by centrifugation, washed repeatedly with ethanol and deionized water, and dried at room temperature to obtain 1DNi-MIL-77.
[0046] (2) The catalyst solution was prepared as follows: the obtained 1D Ni-MIL-77 was put into a mortar and ground into powder, 0.03 g was weighed and put into a Shrek bottle, vacuum-N 2 Replace three times and save for future use. At the same time, put 3...
Embodiment 2
[0048] Example 2 Synthesis and use of 3D Ni-MIL-77 catalyst
[0049] (1) The synthesis method of 3DNi-MIL-77 material is as follows: 0.03mol glutaric acid, 0.02mol NiCl 2 ·6H 2 O and 0.04mol KOH were dissolved in 22ml of a mixed solvent with a volume ratio of ethanol and water of 1:1 (the molar ratio of each reactant was 1.5:1:2:60), fully stirred to dissolve, and the mixed solution was transferred to water The reaction was carried out at 180 °C and autogenous pressure for 48 hours in a hot kettle, and then the reaction kettle was taken out and cooled to room temperature naturally. The obtained product was collected by centrifugation, washed with deionized water until neutral, and dried at room temperature to obtain 3DNi-MIL-77.
[0050] (2) The catalyst solution was prepared as follows: the obtained 3D Ni-MIL-77 was put into a mortar and ground into powder, 0.03 g was weighed and put into a Shrek bottle, activated under vacuum at 150°C for 12 h, taken out and vacuum-N 2 Rep...
Embodiment 3
[0052] Example 3 Influence of the amount of cocatalyst on the effect of 1D Ni-MIL-77 catalyzed by ethylene oligomerization
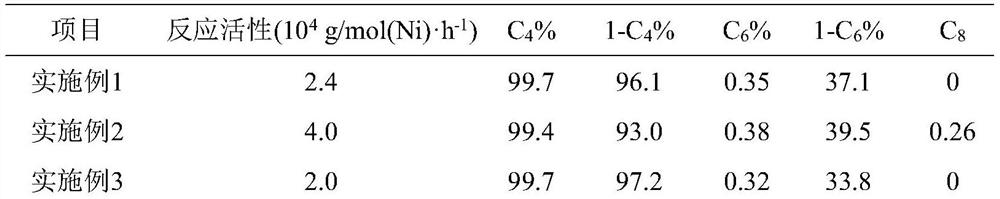
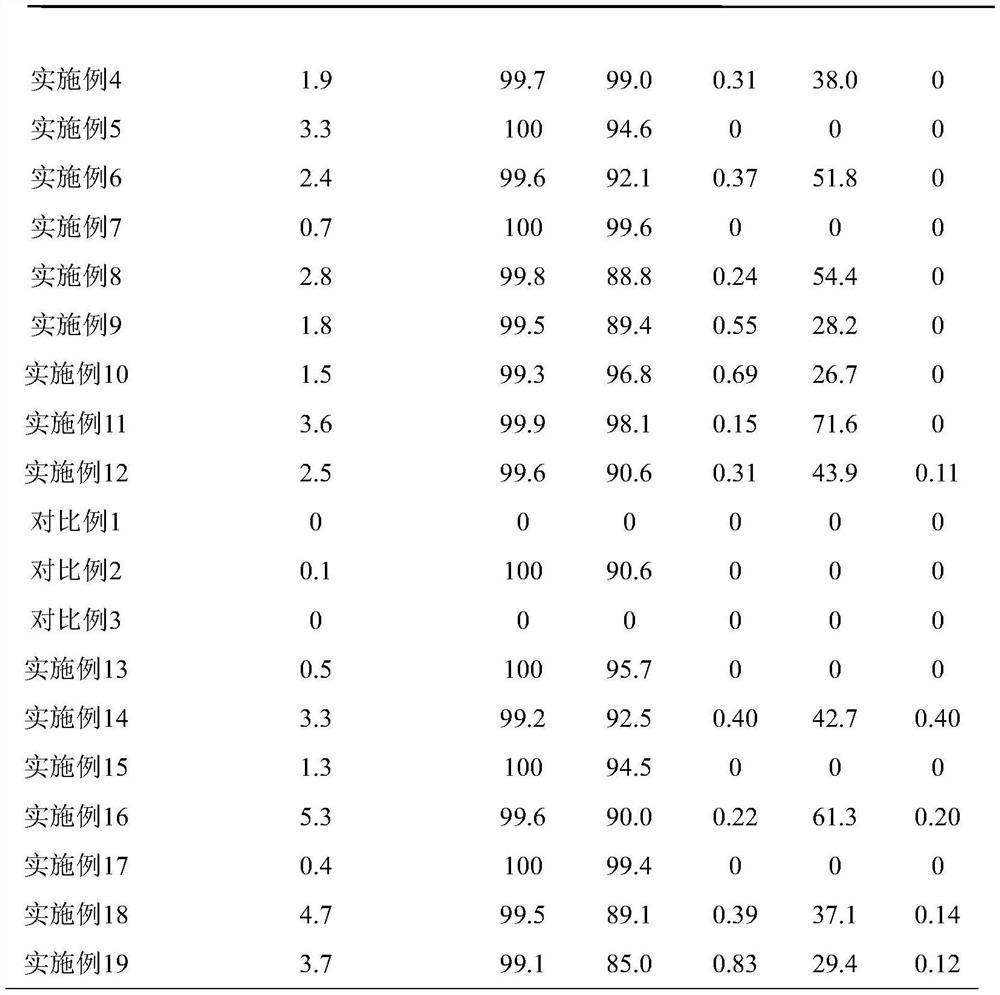
[0053] The difference between this example and Example 1 is that when 1D Ni-MIL-77 catalyst is used in the ethylene oligomerization reaction, the amount of cocatalyst MAO is changed, that is, 3400 μl MAO solution ( Al:Ni=100). The reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com