Preparation method of silver tetrafluoroborate
A technology of silver tetrafluoroborate and tetrafluoroborate, applied in tetrafluoroboric acid, boron halide compounds, etc., can solve problems such as high production cost, easy decomposition, contact with air, etc., and achieve production safety guarantees and mild reaction conditions. , the effect of low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] One embodiment of the present invention provides a method for preparing silver tetrafluoroborate, comprising the following steps: mixing and reacting a fluoroboric acid source and a silver salt in a reaction medium to obtain silver tetrafluoroborate.
[0030] Wherein, the fluoroboric acid source is selected from at least one of tetrafluoroborate and tetrafluoroboric acid; the reaction medium is a mixed solvent of the first organic solvent and water, or water, and the first organic solvent is selected from ether, nitromethane and at least one of toluene.
[0031] The present invention uses the above-mentioned fluoroboric acid source and silver salt as raw materials, and uses a specific type of mixed solvent of the first organic solvent and water as the reaction medium, so that the fluoroboric acid source and silver salt can be mixed to form a macroscopically homogeneous solution for ionization. Exchange and metathesis reaction occurs, and finally form silver tetrafluorob...
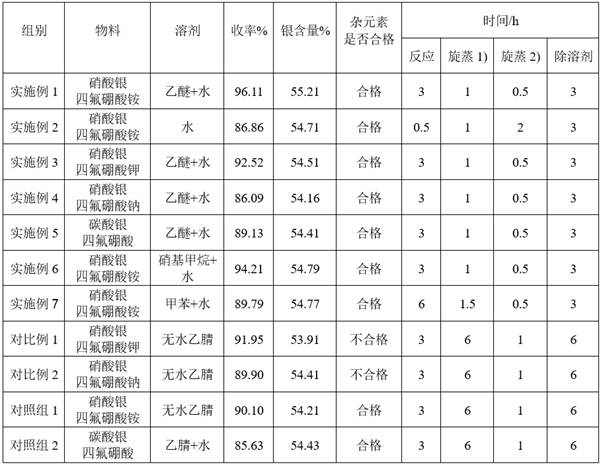
Embodiment 1
[0056] Dissolve 16.98 g of silver nitrate solid in 150 mL of ether to obtain an ether solution of silver nitrate.
[0057] Dissolve 10.58 g of ammonium tetrafluoroborate solid (molar ratio of silver nitrate to ammonium tetrafluoroborate: 1:1.01) in 25 mL of deionized water to obtain ammonium tetrafluoroborate aqueous solution.
[0058] Ammonium tetrafluoroborate aqueous solution was added dropwise to silver nitrate ether solution at room temperature, and stirred for 3 h until the reaction was completed. Filter the solid-liquid mixture obtained by the reaction, wash the filter cake with anhydrous diethyl ether, collect the filtrate, and spin-steam the obtained filtrate at room temperature for 1 h under weak light to remove ether, then continue to heat up to 90 ° C and spin-steam for 0.5 h to remove residual water, and obtain a white Crystal, that is, silver tetrafluoroborate crude product.
[0059] Dissolve the obtained silver tetrafluoroborate crude product in 30 mL of anhydr...
Embodiment 2
[0063] Dissolve 16.98 g of silver nitrate solid in 150 mL of water to obtain a silver nitrate solution.
[0064] Dissolve 10.58 g of ammonium tetrafluoroborate solid (molar ratio of silver nitrate to ammonium tetrafluoroborate: 1:1.01) in 25 mL of deionized water to obtain ammonium tetrafluoroborate aqueous solution.
[0065] Add the ammonium fluoroborate solution dropwise to the silver nitrate solution at room temperature in the dark, and stir for 0.5 h until the reaction is complete. Filter the solid-liquid mixture obtained from the reaction, wash the filter cake with 5 mL of deionized water, collect the filtrate and rotate it at 55 °C and -0.1 MPa pressure for 1 h under dark conditions, then at the same pressure at 75 °C for 1 h, and finally 90 °C for 1 h to remove the residual water by rotary evaporation to obtain white silver tetrafluoroborate crude product.
[0066] Dissolve the obtained silver tetrafluoroborate crude product in 30 mL of anhydrous ether, filter to remov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com