Preparation method of linagliptin dimer impurity
A technology for linagliptin dimer and impurities, which is applied in the field of preparation of linagliptin dimer impurities, can solve the problems that the preparation method of linagliptin impurity I is not reported in literature and the like, and achieves a short synthetic method route. , The effect of high reaction yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
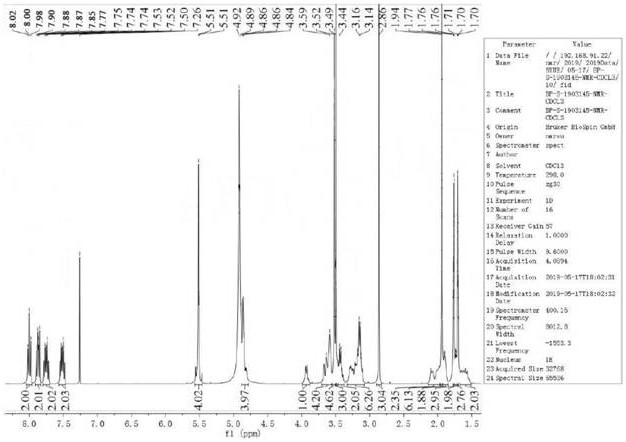
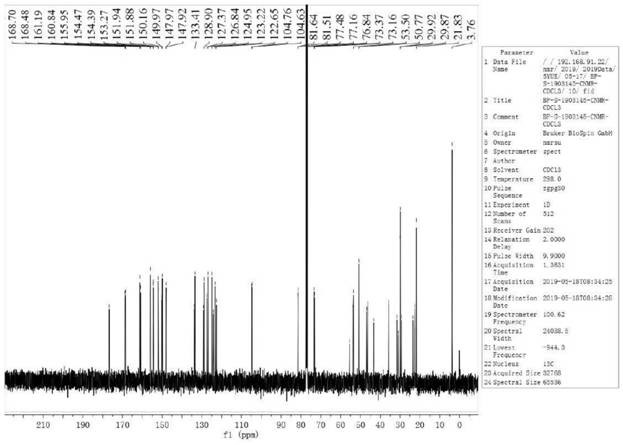
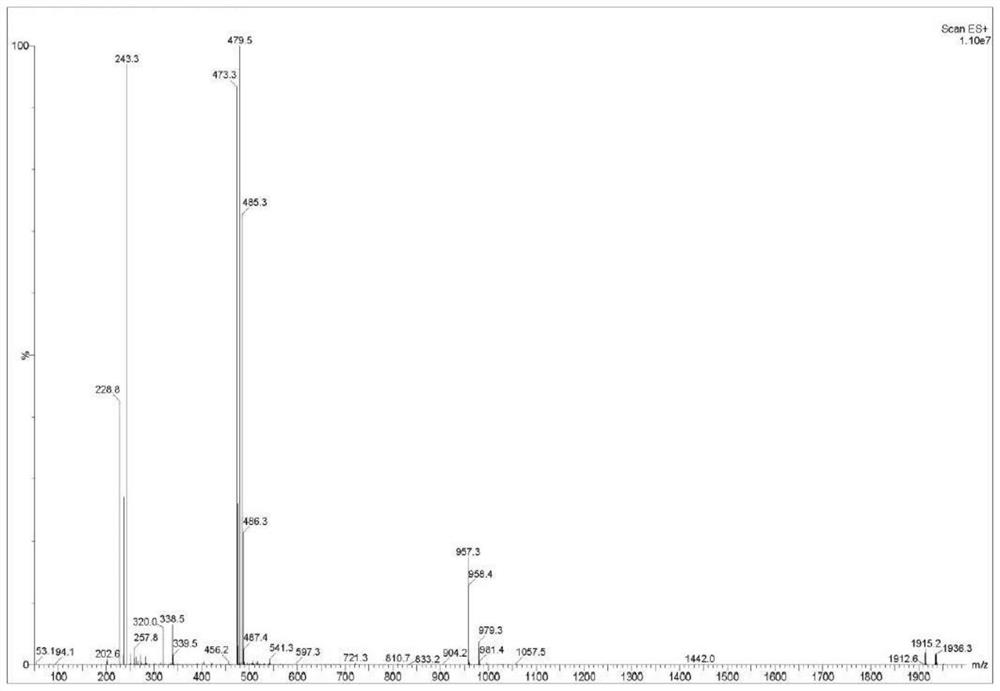
[0037] Compound II, linagliptin (94.51g, 0.2mol) was added into dichloromethane and ethanol (400ml, V 二氯甲烷 :V 乙醇 =10:1) in a mixed solvent of 10:1) and stir to dissolve, add dimethyl azobisisobutyrate (4.61g, 0.02mol) and hydrochloric acid (110ml, 2.0mol / L) successively, heat to 35°C for about 10h, reduce Concentrate under pressure until cloudy, lower the temperature to 15-20°C, add methyl tert-butyl ether (200ml), continue to stir for 1h, filter, rinse the filter cake with cold ethanol (100ml, 0-5°C), and vacuum-dry at 45°C 12h, Liagliptin dimer impurity I was obtained, the yield was 97.12%, and the HPLC purity was 99.91%.
Embodiment 2
[0039] Compound II, linagliptin (94.51g, 0.2mol) was added into dichloromethane and tetrahydrofuran (400ml, V 二氯甲烷 : V 四氢呋喃 = 10:1) in a mixed solvent, stirring and dissolving, adding dimethyl azobisisobutyrate (3.68g, 0.016mol) and formic acid (220ml, 1mol / L) successively, heating to 30°C for about 10h, and depressurizing Concentrate until cloudy, cool down to 15-20°C, add methyl tert-butyl ether (200ml), continue to stir for 1h, filter, rinse the filter cake with cold ethanol (100ml, 0-5°C), and vacuum-dry at 45°C for 12h , the yield of Liagliptin dimer impurity I was 93.40%, and the HPLC purity was 99.82%.
Embodiment 3
[0041] Compound II, linagliptin (94.51g, 0.2mol) was added into dichloromethane and acetonitrile (400ml, V 二氯甲烷 :V 乙腈 =10:1) in the mixed solvent of stirring and dissolving, add dimethyl azobisisobutyrate (23.03g, 0.1mol) and acetic acid (110ml, 2mol / L) successively, heat to 50°C for about 10h, depressurize Concentrate until cloudy, cool down to 15-20°C, add methyl tert-butyl ether (200ml), continue to stir for 1h, filter, rinse the filter cake with cold ethanol (100ml, 0-5°C), and vacuum-dry at 45°C for 12h , the yield of Liagliptin dimer impurity I was 89.85%, and the HPLC purity was 99.31%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com