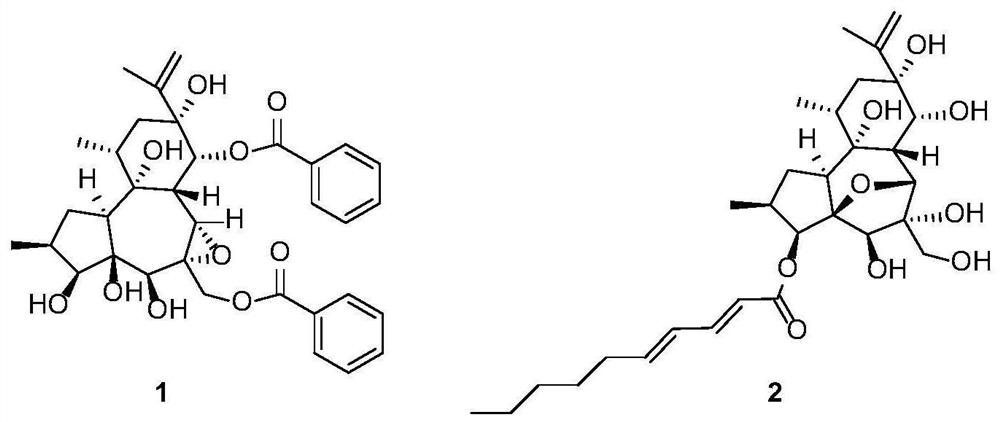
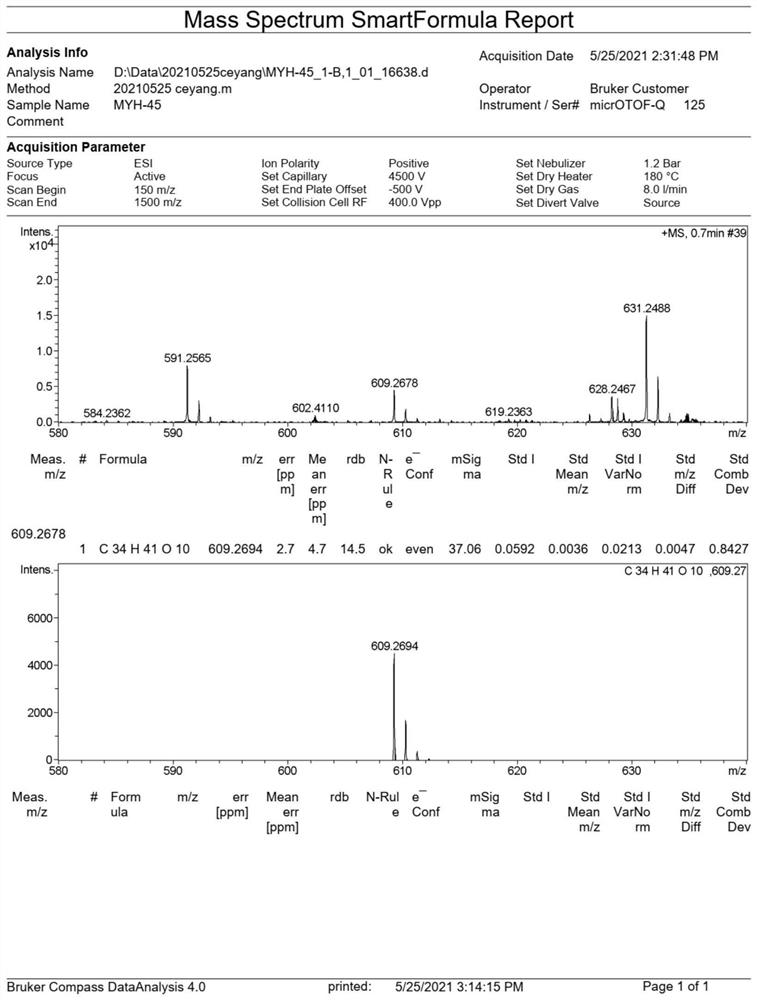
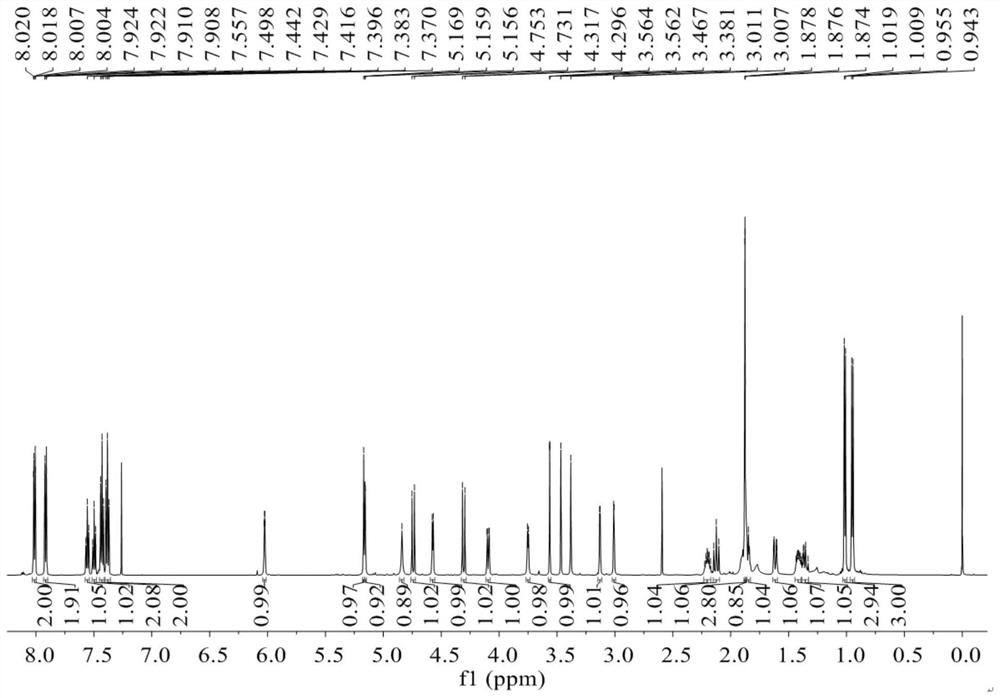
Preparation and application of daphnane diterpenoid compounds in flos genkwa flower bud
A technology of daphne-type diterpenes and compounds, applied in the field of medicine, can solve problems such as no patents or literature reports, and achieve good anti-tumor cell growth activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation of daphne-type diterpenoids 1-2 in Daphne genkwa flower buds specifically includes the following operations:
[0054] (1) Dried Daphne genkwa flower buds (60 kg) were reflux extracted with 70% industrial ethanol for 3 times, and the combined extracts were concentrated to obtain extracts. The obtained extract was successively extracted with dichloromethane and ethyl acetate. The dichloromethanol layer part (2400g) and the ethyl acetate layer part (1300g) were subjected to vacuum silica gel column chromatography, and the dichloromethane-methanol system (100:1, 50:1, 30:1, 20:1, 10: 1, 5:1, 3:1, 1:1) for gradient elution, and a total of 4 fractions (Fr.A~Fr.D) were collected.
[0055] (2) Fraction Fr.C (230g) was chromatographed on HP20 column, eluted with ethanol water gradient (20%, 40%, 60%, 90%), and a total of 4 fractions (Fr.C-1~Fr. C-4).
[0056] (3) 4 fractions Fr.C-1 (25g), Fr.C-2 (32g), Fr.C-3 (35g), Fr.C-4 (43g) were respectively subjected to ...
Embodiment 2
[0059] Examination of antitumor cell activity of daphne-type diterpenoids 1-2 in Daphne genkwa flower buds.
[0060] The growth inhibitory activity of compound 1-2 prepared in Example 1 on tumor cell lines A549, Hep3B, and MCF-7 was tested by MTT method. Take logarithmically grown cells in 6×10 4 cells / mL, inoculated in a 96-well plate with 100 μL per well, set up three replicate wells and placed at 37°C, 5% CO 2 Incubate in the incubator for 24h. Then they were treated with different concentrations of test compounds 1 and 2 (0.1, 1, 10, 50 μM) for 48 hours.
[0061] After 48 hours of drug action, the cells were incubated with MTT solution (0.5 mg / mL) at 37° C. for another 4 hours. After aspirating the culture solution, DMSO was added to each well to dissolve it completely, and the absorbance was measured at 490 nm with a microplate reader. All experiments were carried out in parallel and repeated three times, and the results of blank group and positive control group were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com