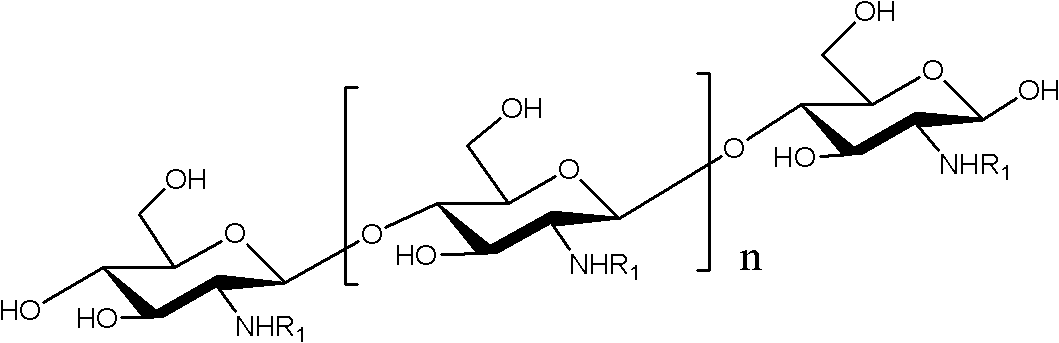
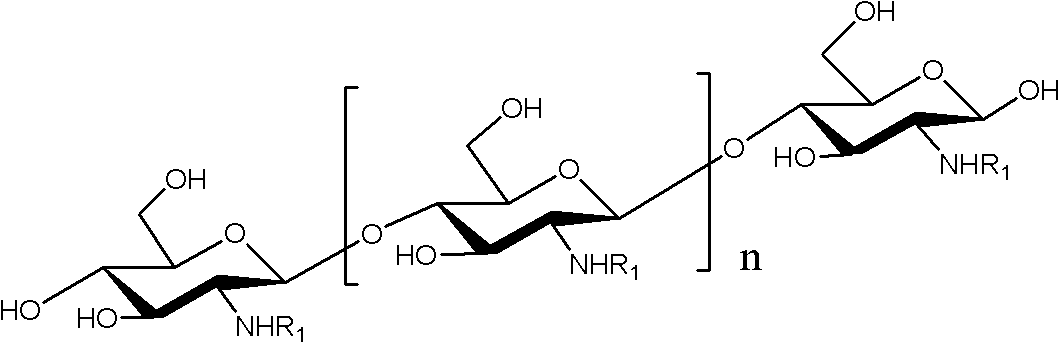
N-unsaturated fatty acid acylated chitosan oligosaccharide and preparation and application thereof
A technology for fatty acid acylation of chitosan oligosaccharide and unsaturated fatty acid, which is applied in the field of N-unsaturated fatty acid acylation chitosan oligosaccharide and its preparation, and can solve the problems of insufficient activity, troublesome use and storage, and insufficient stability of unsaturated fatty acid. , to achieve the effect of simple operation process, mild conditions and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of N-linoleic acid acylated chitosan oligosaccharide (ammonolysis method):
[0032] Dissolve 2.8g of linoleic acid in N,N-dimethylformamide (DMF), add 2.7g of p-nitrophenol, stir, add 0.1g of dimethylaminopyridine, dropwise add 2.06g of dicyclohexylcarbodiene Dissolve the imine in 5mL of DMF, react under heating at 60-70°C for 2-6 hours after dripping, filter, pour the filtrate into ice water, precipitate white crystals, filter, and recrystallize with absolute ethanol to obtain linoleic acid 2.0g p-nitrophenyl ester, aminolysis of ester:
[0033] Get 1.28g (3.2mmol) of p-nitrophenyl linoleate and dissolve it in 30mL of anhydrous dimethyl sulfoxide, and add 0.6g (3.1mmol) of chitosan oligosaccharide with a degree of polymerization of 2-8 (the mole of ester and oligosaccharide The ratio is 1-2:1 (calculated according to the glucosamine residue in chitosan oligosaccharide), react at 60-70°C for 4 hours, add ether / acetone solution 50-100mL (1:...
Embodiment 2
[0036] Embodiment 2: the preparation of N-linoleic acid acylated chitosan oligosaccharide (one-step method)
[0037] Take 1.62g of chitosan oligosaccharide with a degree of polymerization of 2-15 and dissolve it in 30mL of N,N-dimethylformamide, then add 0.1g of dimethylaminopyridine, add 4mL of methanol; take 3.0g of linoleic acid and dissolve it in Add N,N-dimethylformamide to the above solution; after reacting at 60°C for 4 hours, add 5 times the volume of ethanol to the reaction solution to produce a large amount of yellow precipitate, suction filter, and filter the cake with anhydrous Wash with acetone for 3 times to obtain a yellow powder, which is vacuum-dried to obtain N-linoleic acid acylated chitosan oligosaccharide powder as a solid. The infrared data are identical or substantially identical to Example 1. The degree of substitution was determined to be 71% by nuclear magnetic resonance.
Embodiment 3
[0038] Embodiment 3: the preparation of N-eicosapentaenoic acid acylated chitosan oligosaccharide (one-step method)
[0039]Take 1.62g chitosan oligosaccharide and dissolve it in 30mL of N,N-dimethylformamide, then add 0.1g of dimethylaminopyridine, add 4mL of methanol; take 3.5g of linoleic acid and dissolve it in N,N-dimethylformamide Formamide was added to the above solution; after reacting at 60°C for 4 hours, 5 times the volume of ethanol was added to the reaction solution to produce a large amount of light yellow precipitate, which was filtered by suction, and the filter cake was washed 3 times with anhydrous acetone to obtain Yellow powder, vacuum-dried to obtain N-eicosapentaenoic acid acylated chitosan oligosaccharide powder solid. Infrared spectrum (KBr) main absorption peak (IRu, cm -1 ): 3580~3390 (OH, NH); 3095 (HC=CH) 2939; 2860 (CH+CH 2 ); 1705 (C=C); 1685 (amide-I band); 1650 (amide-II band); 1116, 1054, 1033 (C-O); 891 (β, C-H). Same; The degree of substitu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com