Method for producing porcine pseudorabies gE gene deletion virus inactivated vaccine
A porcine pseudorabies, gene deletion technology, applied in the fields of biochemical equipment and methods, vaccines, viruses, etc., can solve the problems of lack of in-depth research, hidden dangers of vaccine safety, and long virus liquid time, so as to shorten production time and improve immunity. effect, the effect of improving growth activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
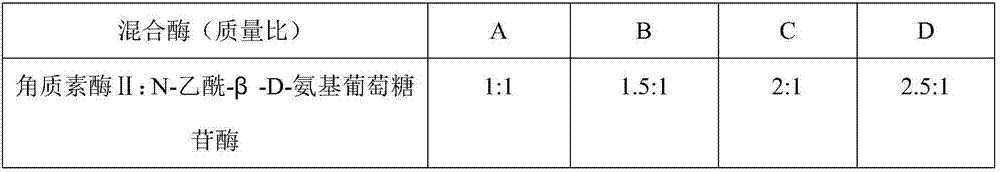
[0029] Embodiment 1 Keratan sulfate oligosaccharide preparation and component analysis
[0030]
[0031] Preparation of Group A:
[0032] Take 50g of keratan sulfate, dissolve it in 300ml of 0.1M acetate buffer (pH6.0), add 25U of mixed enzyme, and degrade at 37°C for 24h. After the reaction, 2 times the volume of ethanol was added, stirred, left overnight at room temperature, centrifuged at 4000 rpm for 15 min, and the supernatant (supernatant A) was taken. Add 300ml of distilled water to the precipitate to dissolve, add 3 times the amount of ethanol, stir, leave at room temperature overnight, centrifuge at 4000rpm for 15min, and take the supernatant (supernatant B). Supernatant A and supernatant B were mixed, concentrated under reduced pressure, and used Bio-Gel-P-2 column (3.6 ╳ 134cm), using distilled water as solvent, carry out gel filtration, and freeze-dry the filtrate to get final product.
[0033] The preparation of keratan sulfate oligosaccharides in groups B-D...
Embodiment 2
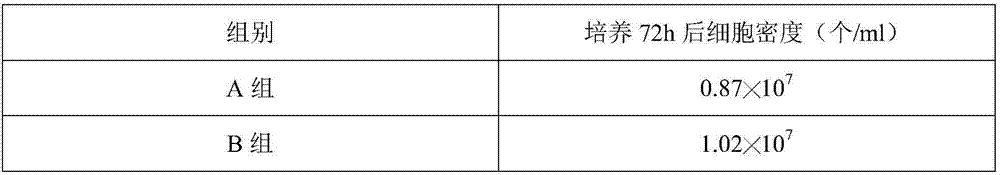
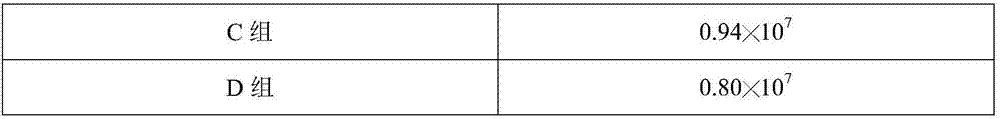
[0038] The influence of embodiment 2 keratan sulfate oligosaccharides on BHK-21 cell growth
[0039] BHK21 cells were cultured with keratan sulfate oligosaccharides prepared in Example 1A-D groups, specifically:
[0040] (1) Take the BHK21 cell species out of the liquid nitrogen tank for recovery, add it to DMEM medium containing 10% newborn bovine serum, and store it at 37°C, 5% CO 2 cultured until it grows into a good monolayer, then digested with an appropriate amount of trypsin digestion solution containing 0.02% EDTA, digested at 37°C for 6min, and adjusted the cell density to 2.26×10 with DMEM medium containing 10% newborn bovine serum. 5 cell suspension per mL;
[0041] (2) Put the cell suspension obtained in step (1) into a spinner bottle, add cell growth solution, the volume ratio of the cell suspension to the cell growth solution is 1:10, and the cell growth solution contains 4 mmol / L glutamine, 0.8mg / L dilinoleoylphosphatidylcholine, 4% (m / v) D-glucosamine, 2% (m...
Embodiment 3
[0047] Example 3 Production of porcine pseudorabies gE gene deletion virus with BHK-21 cells
[0048] (1) Take the BHK21 cell species out of the liquid nitrogen tank for recovery, add it to DMEM medium containing 10% newborn bovine serum, and store it at 37°C, 5% CO 2 cultured until it grows into a good monolayer, and then digested with an appropriate amount of trypsin digestion solution containing 0.02% EDTA, digested at 37°C for 6 minutes, and adjusted the cell density to 3.24×10 with DMEM medium containing 10% newborn bovine serum. 5 cell suspension per mL;
[0049] (2) Put the cell suspension obtained in step (1) into a spinner bottle, add cell growth solution, the volume ratio of the cell suspension to the cell growth solution is 1:10, and the cell growth solution contains 6 mmol / L glutamine, 0.4mg / L dilinoleoylphosphatidylcholine, 4% (m / v) D-glucosamine, 3% (m / v) growth promoter, 1.0% (v / v) double antibody The DMEM culture solution was placed on a bottle spinner and c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com