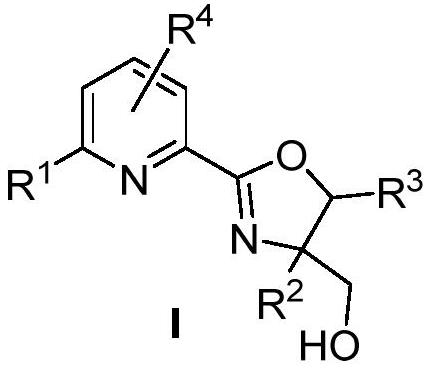
Hydroxyl-substituted pyridine oxazoline ligand and application thereof in hydrohalogenation reaction of olefin
A technology of pyridine oxazoline and ligand, which is applied in the field of hydroxyl-substituted pyridine oxazoline ligand and its application in the hydrohalogenation reaction of olefins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] The preparation of the pyridine-oxazoline ligand that embodiment 1 hydroxyl replaces
[0134]
[0135] by As an example, the synthetic operation steps of hydroxy-substituted pyridine oxazoline are introduced:
[0136] Add substituted pyridine S1 (13.5g, 100mmol, 1.0equiv.) and dichloromethane (150mL) into a 500mL reaction flask, add m-chloroperoxybenzoic acid (32.5g, 150mmol, 1.5equiv.) in batches under stirring, Reaction at room temperature, monitored by TLC. After the reaction, add K 2 CO 3 (27.6g, 200mmol, 2.0equiv.), stirred at room temperature for 30min, then filtered under reduced pressure, and the filtrate was spin-dried to obtain pyridine nitrogen oxide compound S2, which was directly put into the next reaction.
[0137] Add S2, dichloromethane (150mL), trimethylsilyl cyanide (14.4g, 150mmol, 1.5equiv.) into a 100ml egg-shaped flask, stir at room temperature for 15 minutes, then add N,N-dimethylcarbamoyl chloride (15.6 g, 150mmol, 1.5equiv.), stirred ov...
Embodiment 2
[0159] The hydrochlorination reaction of embodiment 2 palladium catalysis olefin
[0160]
[0161] Pd(CH 3 EN) 2 Cl 2 (6.5mg, 0.025mmol, 5mol%), L1 (9.2mg, 0.0375mmol, 7.5mol%), and NCS (133.3mg, 1.0mmol, 2.0equiv.) were weighed into the sealed tube in turn and pumped to keep the system in Under nitrogen atmosphere, then sequentially add the dry mixed solvent CH 3 CN:CH 2 Cl 2 (2:8, 5.0mL), alkene substrate (0.5mmol, 1.0equiv), water (10.0μL), triethylamine (25μL, 1M in DCM, 0.025mmol, 5mol%) and triisopropylsilane ( 0.3mL, 1.5mmol, 3.0equiv), stirred at room temperature until complete. After the reaction finishes, pass through a silica gel short column (300-400 mesh, CH 2 Cl 2 Elution), the solvent was spin-dried, and the product was obtained by column chromatography (300-400 mesh, PE:EA elution).
[0162]
[0163] Colorless liquid (111.7mg, 89% yield). 1 H NMR (400MHz, CDCl 3 )δ7.82–7.80(m,2H),7.71–7.68(m,2H),3.67(t,J=7.6Hz,2H),3.50(t,J=6.8Hz,2H),1.83–1.76(m...
Embodiment 3
[0270] The hydrobromination reaction of embodiment 3 palladium catalysis olefin
[0271]
[0272] Pd(hfacac) 2 (26.0mg, 0.05mmol, 10mol%), L3 (17.5mg, 0.075mmol, 15mol%), LiClO 4 (53.4mg, 0.5mmol, 1.0equiv) or HCl (50μL, 40mol%, 4.0M in dioxane), Na-NMBI (229.0mg, 1.0mmol, 2.0equiv) were weighed into the sealed tube in sequence and pumped to keep the system in Under nitrogen atmosphere, then sequentially add the dry mixed solvent CH 3 CN:CH 2 Cl 2 (4:6, 5.0mL), alkene substrate (0.5mmol, 1.0equiv), H 2 O (4.0 μL for HCl, 10 μL for LiClO 4 ) and triisopropylsilylhydrogen (0.22mL, 1.1mmol, 2.2equiv), stirred and reacted at room temperature. After 6 hours, Na-NMBI (114.5 mg, 0.5 mmol, 1.0 equiv) and triisopropylsilylhydrogen (0.11 mL, 0.55 mmol, 1.1 equiv) were added to continue the reaction until complete. After the reaction finishes, pass through a silica gel short column (300-400 mesh, CH 2 Cl 2 Elution), the solvent was spin-dried, and the product was obtained by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com