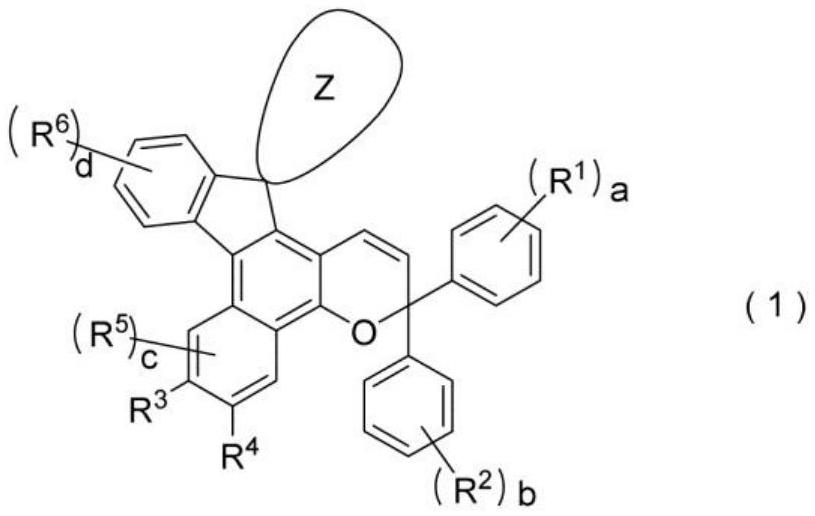
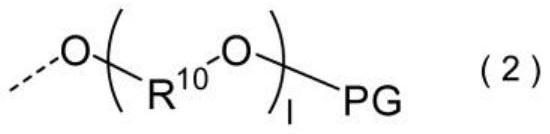
Chromene compound and photochromic optical article
A technology of chromene compounds and polymerizable groups, which is applied in the field of new photochromic optical articles, can solve problems such as adverse effects on the eyes, achieve high degree of polymerization, and inhibit dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0308] 1.0 g (2.8 mmol) of the naphthol compound represented by the following formula (19)
[0309]
[0310] 10 mass % methyl ethyl ketone solution 12.0 g (3.4 mmol) of a propargyl alcohol compound having a reactive substituent (hydroxyl group) represented by the following formula (20)
[0311]
[0312] This was dissolved in 50 mL of toluene, 0.02 g of p-toluenesulfonic acid was further added, and the mixture was stirred under heating and reflux for 1 hour. After the reaction, the solvent was removed, and purification was performed by silica gel chromatography to obtain 1.4 g of a white powdery product (precursor). The yield was 72%.
[0313] The obtained precursor was dissolved in 0.7 g (6.9 mmol) of triethylamine and 30 mL of dichloromethane, and ice-cooled. There, 0.3 g (2.9 mmol) of acryloyl chloride was slowly added dropwise. After the reaction, the solvent was removed, and purification was performed by silica gel chromatography to obtain 1.3 g of a white powdery...
Embodiment 2~10
[0319] In the same manner as in Example 1, the chromene precursors and chromene compounds shown in Tables 4 to 6 were synthesized using the naphthol compounds shown in Tables 1 to 3 and the propargyl alcohol compound having a reactive substituent. As a result of structural analysis of the obtained product using the same structure confirmation method as in Example 1, it was confirmed that it was a compound represented by the structural formulas shown in Tables 4-6. In addition, Table 7 shows the elemental analysis values of these compounds, the calculated values obtained from the structural formulas of each compound, and 1 Characteristic spectrum of H-NMR spectrum.
[0320] [Table 1]
[0321]
[0322] [Table 2]
[0323]
[0324] [table 3]
[0325]
[0326] [Table 4]
[0327]
[0328] [table 5]
[0329]
[0330] [Table 6]
[0331]
[0332] [Table 7]
[0333]
Embodiment 11~22
[0335] (Physical property evaluation of produced photochromic contact lenses (photochromic optical articles))
[0336] As the photochromic curable composition, tris(trimethylsiloxy)silylpropyl methacrylate / dimethylacrylamide / 2-hydroxyethyl methacrylate as radical polymerizable monomers were used / Ethylene glycol dimethacrylate in the compounding ratio of 60 parts by mass / 30 parts by mass / 8 parts by mass / 2 parts by mass, respectively. With respect to 100 parts by mass of the mixture of radically polymerizable monomers, 1 part by mass of chromene compounds Nos. 1 to 10 obtained in Examples 1 to 10 was added and mixed well, and then 0.3 parts by mass of thermal polymerization initiator V65 was added. (2,2'-azobis(2,4-dimethylvaleronitrile)) and fully mixed to prepare a photochromic curable composition. The obtained photochromic curable composition was injected into a mold composed of a glass plate and a polyethylene terephthalate (PTFE) sheet having a film thickness of 0.1 mm, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com