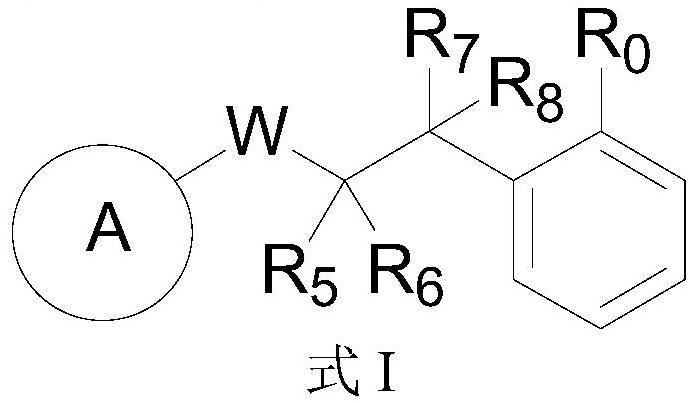
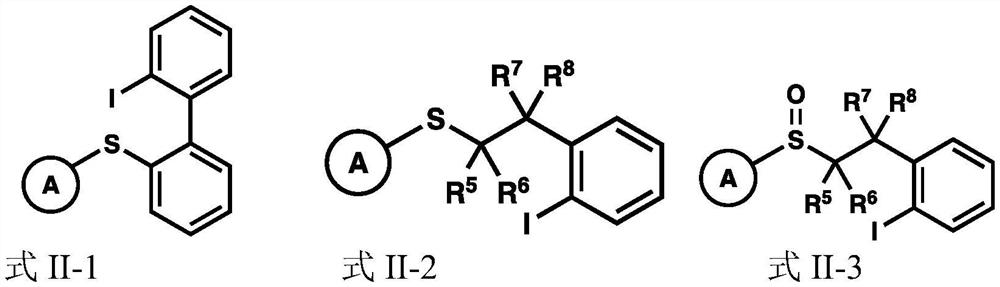
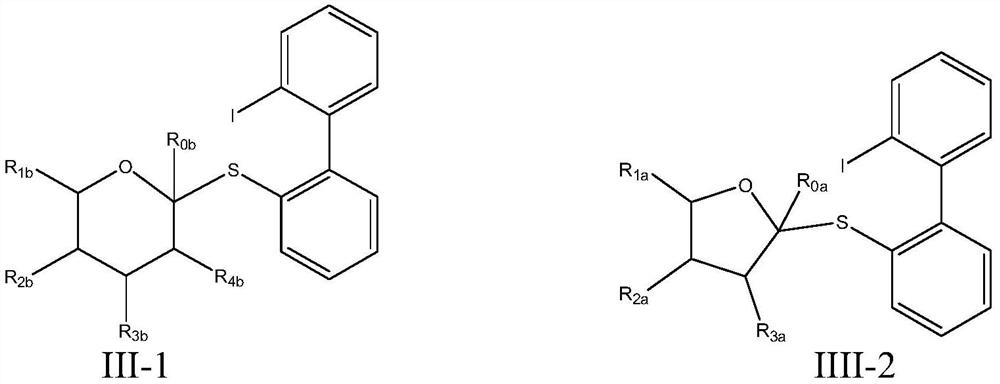
Glycosyl donor and application of glycosyl donor in preparation of glucoside
A donor and sugar-based technology, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of insufficient mild conditions and poor functional group compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] The synthesis of embodiment 1, compound 3
[0093] According to the following synthetic route 1, compound 3 was obtained.
[0094]
[0095] Reaction condition 1:
[0096] Under nitrogen protection, Box ligand (0.1 equiv.) and catalyst (0.05 equiv.) were added to the reaction flask, and then triethylamine (3.0 equiv.) and dry toluene solvent were added. Under nitrogen protection, stir at 80°C for 15 minutes. Then cooled to room temperature, under the protection of nitrogen, the whole Ac-protected mercaptoglucose substrate 1 (1.0 equiv) and periodiodine reagent 2 (1.5 equiv.) were sequentially added and reacted under the protection of nitrogen.
[0097] Reaction condition 2:
[0098] Add all Ac-protected mercaptoglucose substrate 1 (1.0 equiv.) and periodine reagent 2 (1.5 equiv.) into a round bottom flask, add dry toluene, Cu catalyst (0.2 equiv.), and triethyl Amine (3.0 equiv.), heated reaction under nitrogen protection. After the reaction was completed, diatom...
Embodiment 2
[0104] The synthesis of embodiment 2, compound 4
[0105]
[0106] (3R,4R,5S,6S)-2-((2'-iodo-[1,1'-biphenyl]-2-yl)thio)-6-methyltetrahydro-2H-pyran-3,4,5-triyl triacetate
[0107] 1 H NMR (400MHz, CDCl 3 )δ: 7.94 (dd, J = 8.0, 1.3Hz, 1H), 7.69 (dd, J = 7.7, 1.5Hz, 0.5H), 7.62 (dd, J = 7.6, 1.5Hz, 0.5H), 7.45–7.30 (m,3H),7.21(ddd,J=11.0,7.6,1.7Hz,1H),7.15(ddd,J=7.4,4.4,1.7Hz,1H),7.08(tt,J=7.7,1.9Hz,1H ),5.42(d,J=1.7Hz,0.5H),5.38(dd,J=2.9,1.7Hz,0.5H),5.34(d,J=1.7Hz,0.5H),5.24(dd,J=3.2 ,1.6Hz,0.5H),5.17–4.95(m,2H),4.23(dq,J=9.2,6.2Hz,0.5H),4.08–3.98(m,0.5H),2.13(d,J=2.7Hz ,3H),2.03(d,J=5.9Hz,3H),1.96(d,J=2.5Hz,3H),1.20(dd,J=6.2,3.3Hz,3H).
Embodiment 3
[0108] The synthesis of embodiment 3, compound 5
[0109]
[0110] (3S,4R,5R)-2-((2'-iodo-[1,1'-biphenyl]-2-yl)thio)tetrahydro-2H-pyran-3,4,5-triyl triacetate
[0111] 1 H NMR (400MHz, CDCl 3 )δ: 7.92(dd, J=7.9, 1.3Hz, 1H), 7.74(dd, J=7.1, 1.8Hz, 0.6H), 7.70–7.65(m, 0.4H), 7.43–7.31(m, 3H) ,7.22(ddd,J=7.7,6.1,1.7Hz,1H),7.18–7.12(m,1H),7.06(td,J=7.6,1.7Hz,1H),5.26(ddq,J=13.8,5.4, 2.5Hz, 1H), 5.18(t, J=8.3Hz, 0.6H), 5.10(t, J=7.6Hz, 0.4H), 5.01(ddd, J=10.7, 8.2, 3.4Hz, 1H), 4.82( d,J=8.0Hz,0.6H),4.72(d,J=7.1Hz,0.4H),4.08(ddd,J=14.2,12.6,4.5Hz,1H),3.63(ddd,J=17.5,12.6, 2.4Hz, 1H), 2.11(d, J=6.0Hz, 3H), 1.97(d, J=2.1Hz, 3H), 1.91(d, J=8.7Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com