Preparation method of GLP-1 analogue fragment activated ester
A technology of GLP-1 and analogues, which is applied in the field of peptide pharmaceuticals, can solve the problems of unfavorable industrial scale-up production in the preparation of activated esters of GLP-1 analogue fragments, and achieve the effect of shortening the preparation time and avoiding decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
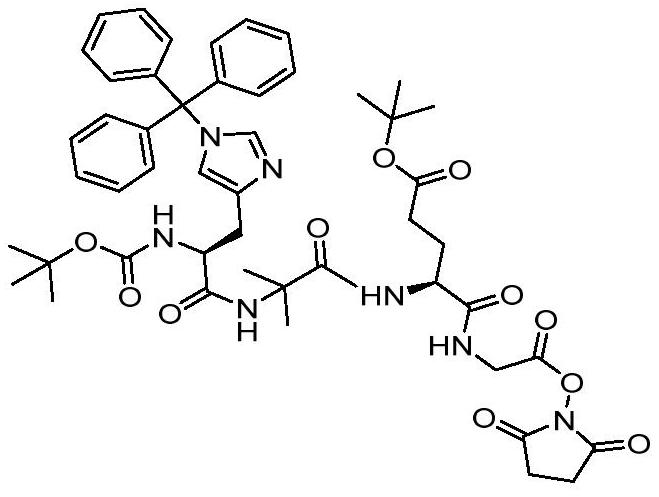
[0031] Example 1: Synthesis of Boc-His(Trt)-Aib-Glu(O-tBu)-Gly-OSu
[0032]
[0033] Weigh 82.5g Boc-His(Trt)-Aib-Glu(O-tBu)-Gly-OH and 2.4g DMAP, add 25.6gDSC, then add 500mL DMF, stir and dissolve, control the reaction temperature 0-5℃, add 19.83ml of DIEA was reacted at 0-10°C for 0.5-1h to obtain Boc-His(Trt)-Aib-Glu(O-tBu)-Gly-OSu with a detection conversion rate of 84%.
Embodiment 2
[0034] Example 2: Synthesis of Boc-His(Trt)-Aib-Glu(O-tBu)-Gly-OSu
[0035] Weigh 82g of Boc-His(Trt)-Aib-Glu(O-tBu)-Gly-OH and 2.4g of DMAP, add 25g of DSC, then add 500ml of DMF, after stirring and dissolving, the reaction system is controlled by an ice-water bath at a temperature of 0 -5℃, add 16.73mlEt 3 N, control the reaction at 0-10° C. for 0.5-1 h to obtain Boc-His(Trt)-Aib-Glu(O-tBu)-Gly-OSu, and the detected conversion rate is 82%.
Embodiment 3
[0036] Example 3: Synthesis of Boc-His(Trt)-Aib-Glu(O-tBu)-Gly-OSu
[0037] Weigh 82.5g Boc-His(Trt)-Aib-Glu(O-tBu)-Gly-OH and 2.4g DMAP, add 25.6gDSC, then add 500ml DMF, after stirring and dissolving, the reaction system is controlled by an ice-water bath. -5°C, add 13.19ml NMM, control the reaction at 0-10°C for 0.5-1h, and obtain Boc-His(Trt)-Aib-Glu(O-tBu)-Gly-OSu, the detected conversion rate is 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com