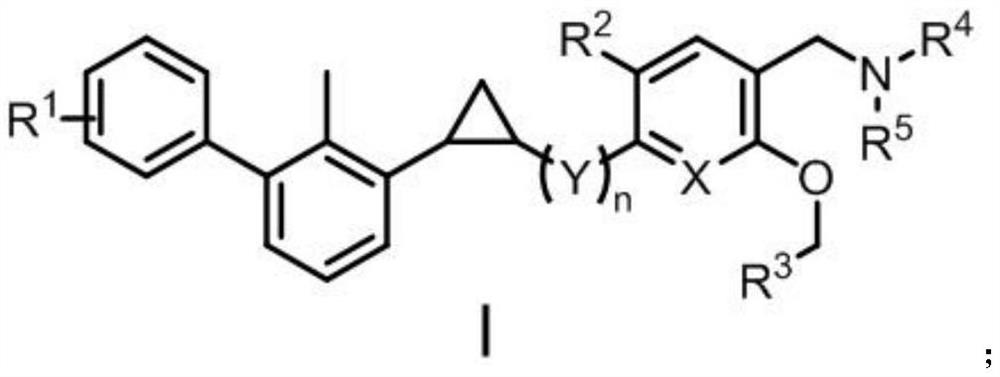
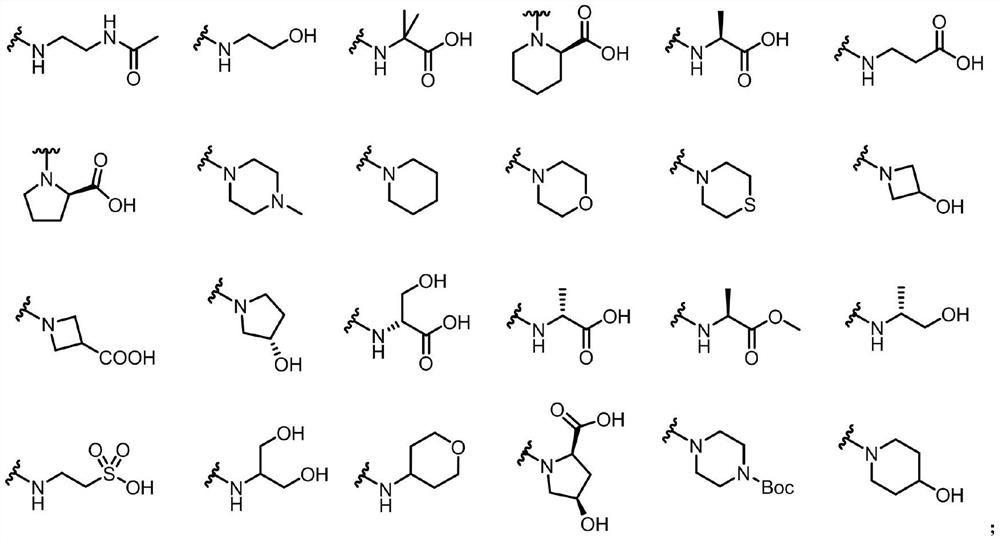
Biphenyl derivative containing cyclopropane structure as well as preparation method and application of biphenyl derivative
A technology of derivatives and biphenyls, applied in the field of biphenyl derivatives and their preparation, can solve the problems of long half-life, poor bioavailability and high immunogenicity, and achieve the effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
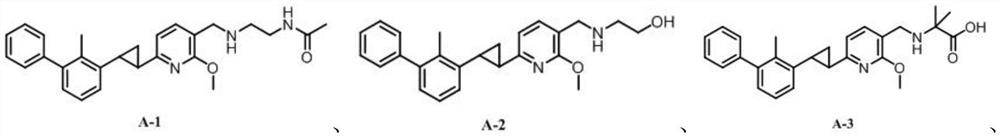
[0066] The synthesis of embodiment 1 compound A-1:
[0067] The synthetic route is as follows:
[0068]
[0069]
[0070] Among them, a:Pd(PPh 3 ) 2 Cl 2 ,CuI,Et 3 N, rt; b: Pd 3 (dba) 2 , Na 2 CO 3 , Dioxane, X-phos, 70°C; c: K 2 CO 3 , MeOH, rt; d: CuCl, NaOtBu, DPEphos rt; e: CH 2 I 2 ,ZnEt 2 , CF 3 COOH,CH 2 Cl 2 ,0℃-rt; f:Pd(PPh 3 ) 4 ,Cs 2 CO 3 , Toluene, 100°C; g: NaBH 3 CN, AcOH, DMF, rt.
[0071] Concrete synthetic method comprises the following steps:
[0072] (1) Synthesis of compound 1-2:
[0073] Bistriphenylphosphine palladium dichloride (Pd(PPh 3 ) 2 Cl 2 , 0.56g, 0.8mmol) and cuprous iodide (CuI, 0.76g, 4mmol) dissolved in 100mL triethylamine (Et 3 N), added 2-chloro-6-iodotoluene (10 g, 40 mmol) under the protection of argon, cooled to 0 ° C, then slowly added trimethylsilylacetylene (12 mL, 80 mmol) with a syringe, and raised to room temperature until The response is complete. After the reaction, suction filtration was perfor...
Embodiment 2
[0085] The synthesis of embodiment 2 compound A-2
[0086] The synthetic route is as follows:
[0087]
[0088] The specific synthesis method is:
[0089] The synthesis of compound A-2 was the same as that of compound A-1 except that the compound ethanolamine was used instead of N-(2-aminoethyl)acetamide. The spectrum information of compound A-2 is as follows:
[0090] 1 H NMR (500MHz, CDCl 3 )δ7.42–7.35(m,3H),7.34–7.27(m,3H),7.20(t,J=7.5Hz,1H),7.16–7.08(m,2H),6.80(d,J=7.3Hz ,1H),3.94(s,3H),3.73(d,J=2.5Hz,2H),3.66(t,J=5.1Hz,2H),2.81–2.71(m,2H),2.60–2.56(m, 1H), 2.21(s,3H), 2.13–2.05(m,1H), 1.75–1.68(m,1H), 1.53–1.43(m,1H).
Embodiment 3
[0091] The synthesis of embodiment 3 compound A-3
[0092] The synthetic route is as follows:
[0093]
[0094] The specific synthesis method is:
[0095] The synthesis of compound A-3 was the same as that of compound A-1, except that compound 2-amino-2-methylpropionic acid was used instead of N-(2-aminoethyl)acetamide. The spectrum information of compound A-3 is as follows:
[0096] 1 H NMR (400MHz, CDCl 3 )δ7.58(d, J=7.5Hz, 1H), 7.37(t, J=7.3Hz, 2H), 7.31(d, J=7.1Hz, 1H), 7.28-7.23(m, 2H), 7.14( t,J=7.5Hz,1H),7.07(t,J=7.6Hz,2H),6.79(d,J=7.3Hz,1H),3.92(s,3H),3.89–3.75(m,2H), 2.59-2.54(m,1H),2.15(s,3H),2.09–1.98(m,1H),1.64(m,1H),1.39(s,6H),0.93–0.77(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com