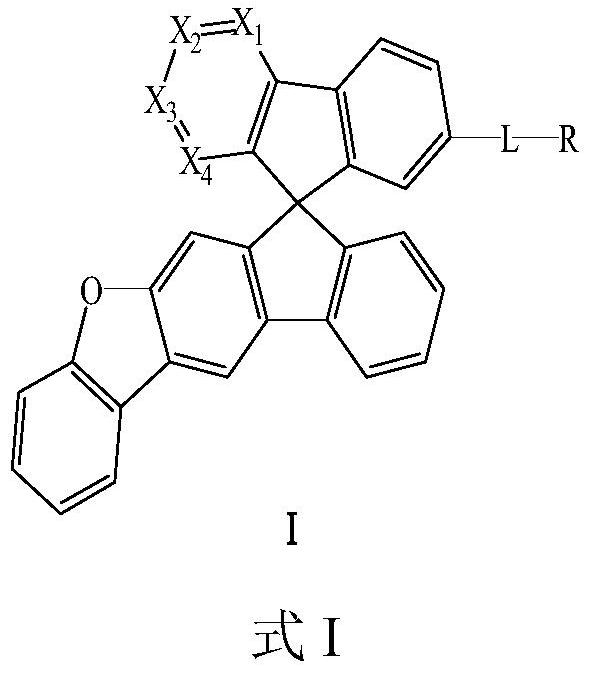
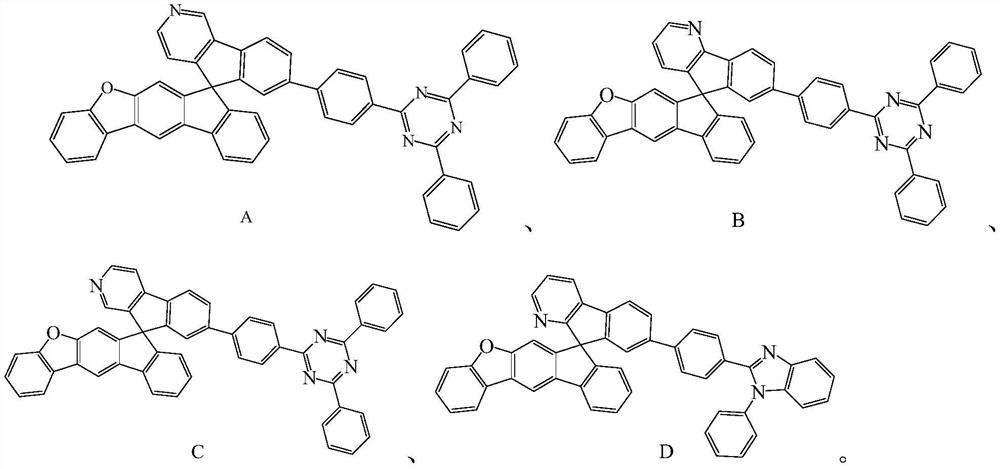
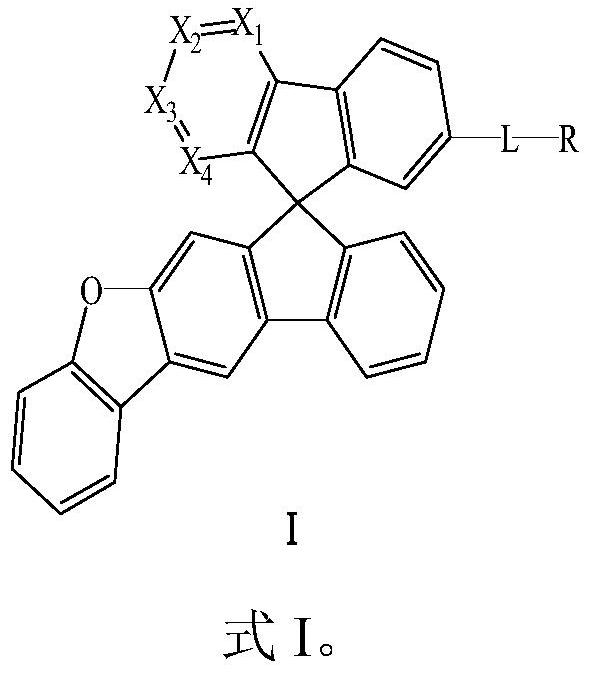
Compound and application thereof
A compound and selected technology, applied in the fields of organic chemistry, electro-solid devices, semiconductor devices, etc., can solve the problems of high power efficiency, long life, and the current efficiency of organic electroluminescence devices needs to be further improved, and achieve high charge transfer capability. , long service life, high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] In this example, compound A was synthesized, and the specific preparation method is as follows:
[0060] step 1):
[0061]
[0062] Specific steps are as follows:
[0063] 486mmol of compound 2, 463mmol of compound 1 and 14mmol of Pd(Ph 3 P) 4 Suspended in 1900ml THF. 463 ml of 2M potassium carbonate solution were slowly added to this suspension and the reaction mixture was heated at reflux for 16 hours. After cooling, the organic phase is separated off, filtered through silica gel, washed three times with 500 ml of water and then evaporated to dryness. Compound 3 was obtained by crystallization from MeOH.
[0064] Step (2):
[0065]
[0066] Specific steps are as follows:
[0067] 64 mmol of compound 4 were introduced into 400 ml THF at -78°C. At this temperature 30 ml of BuLi (2M in hexane) were added dropwise. After 1 hour, 94 mmol of compound 3 in 200 ml of THF was added dropwise. The batch was allowed to stir overnight at room temperature, added to ...
Embodiment 2
[0079] The preparation of Compound B differs from Example 1 in that the synthetic raw materials are different, and it is prepared by a preparation method similar to Compound A.
[0080] The yield of compound B was 78%. Characterization data: melting point (DSC) 286°C, purity 99.9%; 1 H NMR (400MHz, CDCl 3 )δ (ppm): 8.54 (m, 1H), 8.06 (m, 1H), 7.93 (m, 1H), 7.61 (s, 1H), 7.59 (s, 1H), 7.54 (m, 4H), 7.49 ( m,1H),7.48(m,4H),7.44(m,1H),7.42(m,1H),7.41(s,1H),7.38(m,1H),7.37(m,1H),7.32(m ,4H),7.28(m,1H),7.24(m,1H),7.22(m,2H),7.19(m,1H),7.13(m,1H),6.89(m,1H).
Embodiment 3
[0082] The preparation of Compound C differs from Example 1 in that the synthetic raw materials are different, and it is prepared by a preparation method similar to Compound A.
[0083] The yield of compound C was 81%. Characterization data: melting point (DSC) 280°C, purity 99.9%; 1 H NMR (400MHz, CDCl 3 )δ (ppm): 8.63 (m, 2H), 8.06 (s, 1H), 7.61 (s, 1H), 7.59 (s, 1H), 7.54 (m, 4H), 7.51 (m, 1H), 7.49 ( m,1H),7.48(m,4H),7.44(m,1H),7.42(m,2H),7.35(m,1H),7.34(m,1H),7.32(m,4H),7.28(s ,1H),7.24(m,1H),7.22(m,2H),7.19(m,1H),7.13(m,1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com