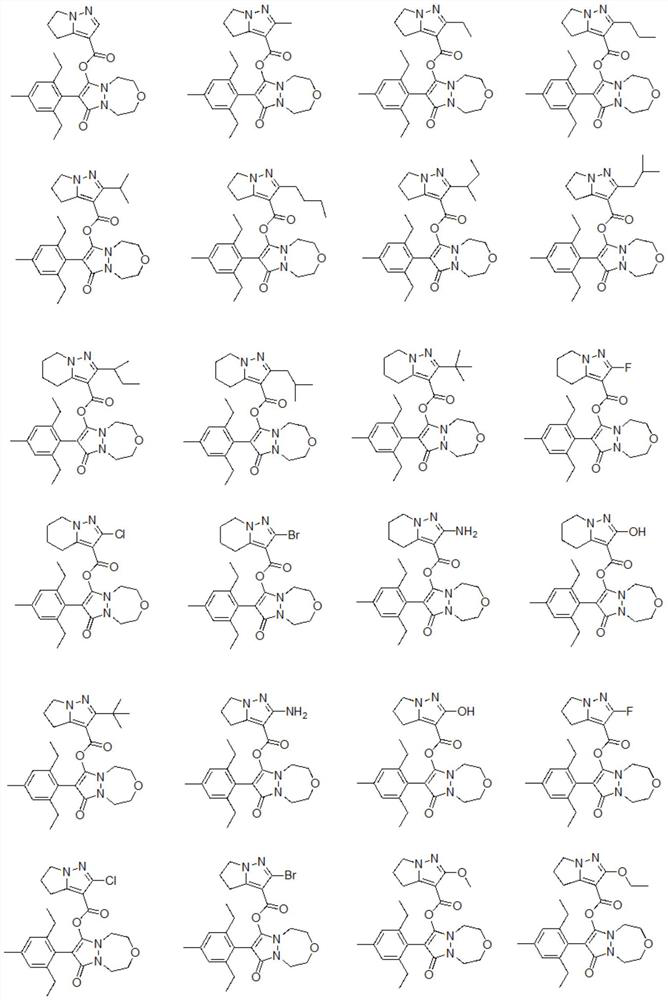
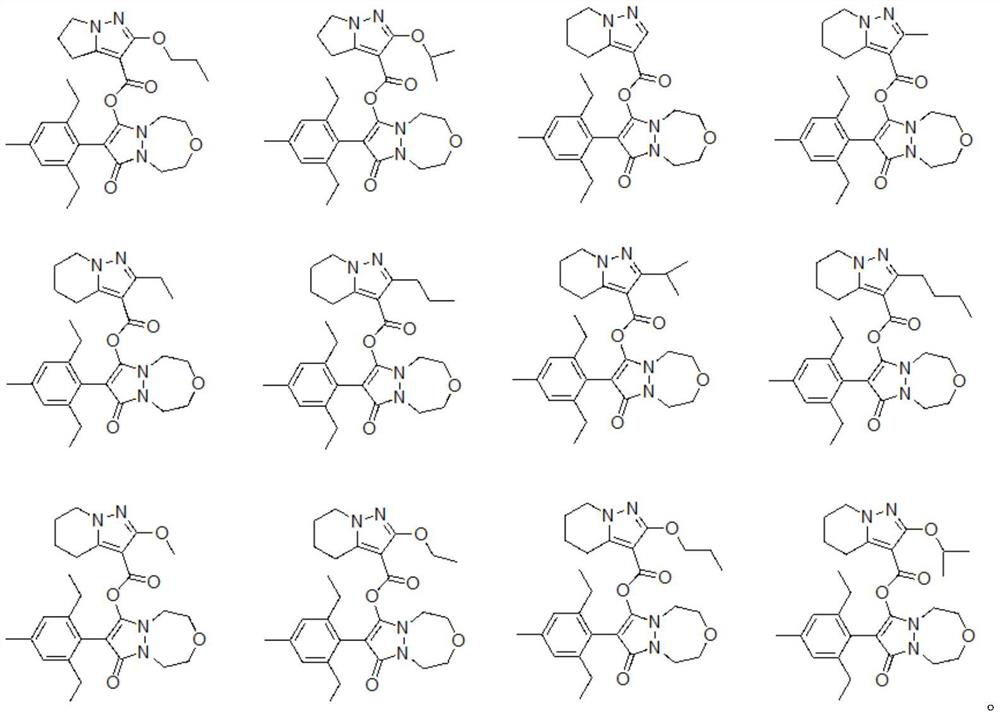
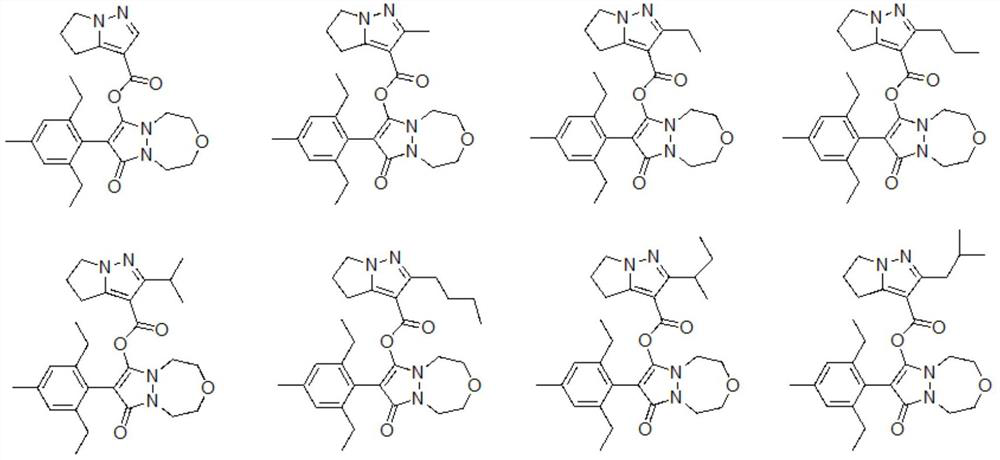
Phenyl pyrazoline derivative or salt, composition and application thereof
A technology of phenylpyrazoline and its derivatives, which is applied in the field of pesticides, can solve the problems of unsatisfactory herbicidal activity and inability to simultaneously kill bacteria and weeds, and achieve the best control effect, good selectivity and safety, Good activity and selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Synthesis of Compound 10:
[0100]
[0101]
[0102] Add intermediate 1 (3.16g, 10mmol) and acetonitrile (30ml) to the reaction flask, add intermediate 2 (1.98g, 10mmol) under stirring at 10°C, stir the reaction at 10°C, add triethylamine (2eq), TLC Follow up the reaction (ethyl acetate:petroleum ether=1:1, GF254, UV color development), after the reaction is complete, spin out the solvent, and purify the compound by column chromatography to obtain the target compound 10, a white solid powder, melting point: 126-128°C ; 1 H-NMR (CDCl 3 -d 6 ,500MHz)δ:6.88(s,2H),4.31-4.28(m,2H),4.05-4.00(t,2H),3.96-3.93(t,2H),3.92-3.89(m,4H),2.78- 2.73(t,2H),2.59-2.52(m,2H),2.48-2.43(m,2H),2.29-2.25(s,6H),1.99-1.93(s,2H),1.79-1.74(s,2H ), 1.13-1.08(m,6H).
Embodiment 2
[0104] Synthesis of Compound 11:
[0105]
[0106] Add intermediate 1 (3.16g, 10mmol) and acetonitrile (30ml) to the reaction flask, add intermediate 3 (2.52g, 10mmol) under stirring at 10°C, stir the reaction at 10°C, add triethylamine (2eq), TLC Follow up the reaction (ethyl acetate:petroleum ether=1:1, GF254, UV color development), after the reaction is complete, spin out the solvent, and purify the compound by column chromatography to obtain the target compound 11, a white solid powder, melting point: 138-141°C ; 1 H-NMR (CDCl 3 -d 6 ,500MHz)δ:6.87(s,2H),4.33-4.30(t,2H),4.06-4.02(t,2H),3.95-3.92(t,2H),3.67-3.63(m,4H),2.72- 2.68(t,2H),2.67-2.63(m,4H),2.53-2.49(s,3H),1.97-1.93(m,2H),1.77-1.74(m,2H),1.15-1.11(m,6H ).
Embodiment 3
[0108] Synthesis of Compound 12:
[0109]
[0110] Add intermediate 1 (3.16g, 10mmol) and acetonitrile (30ml) to the reaction flask, add intermediate 4 (2.40g, 10mmol) under stirring at 10°C, stir the reaction at 10°C and add triethylamine (2eq), TLC Follow up the reaction (ethyl acetate:petroleum ether=1:1, GF254, UV color development), after the reaction is complete, spin out the solvent, and purify the compound by column chromatography to obtain the target compound 12, a light yellow solid powder, melting point: 133-136 ℃; 1 H-NMR (CDCl 3 -d 6 ,500MHz)δ:6.88(s,2H),4.35-4.31(t,2H),4.05-4.01(t,2H),3.93-3.90(t,2H),3.68-3.64(m,4H),2.73- 2.69(t,2H),2.67-2.63(m,4H),2.54-2.50(s,3H),1.98-1.93(m,2H),1.78-1.74(m,2H),1.26-1.22(s,9H ), 1.16-1.11(m,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com