MiRNA simulant and application thereof
A technology of mimics and drugs, applied in the field of miRNA mimics, can solve the problems such as the application of miRNA24-3p mimics as corneal epithelial wound healing drugs, to achieve high commercialization potential and transformation application prospects, accelerate epithelial wound healing, The effect of accelerating corneal re-epithelialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
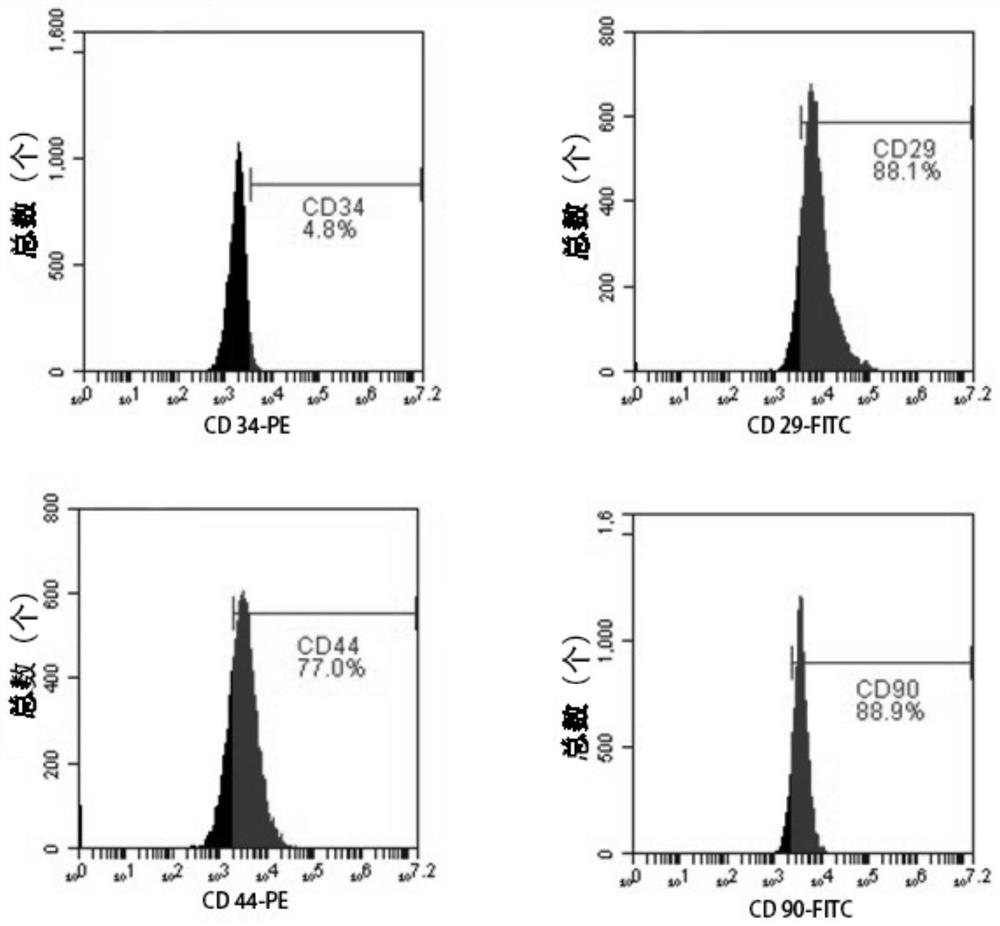
[0030] (1) Adipose-derived mesenchymal stem cells derived from inguinal subcutaneous adipose tissue of New Zealand white rabbits (purchased from Guangdong Provincial Medical Experimental Animal Center) were selected for isolation, culture and passage
[0031] a) The New Zealand white rabbits were killed by euthanasia, and the subcutaneous adipose tissue of the inguinal part of the rabbits was taken under aseptic conditions, and the PBS buffer (pH7.4, In Gibco company, the same below;) repeated washing 3 times;
[0032] b) Use ophthalmic scissors and ophthalmic forceps to remove fascia and blood vessels, rinse with pre-cooled PBS buffer 3 times, take the fat globules and gently transfer them to a sterile plate, and then add 2 times the volume to dissolve them in PBS buffer 1% collagenase type II (purchased from SIGMA, USA) solution in the solution, and placed in a 37°C incubator, and the plate was taken out every 10min and gently shaken, and the total digestion time was 120min;...
Embodiment 2
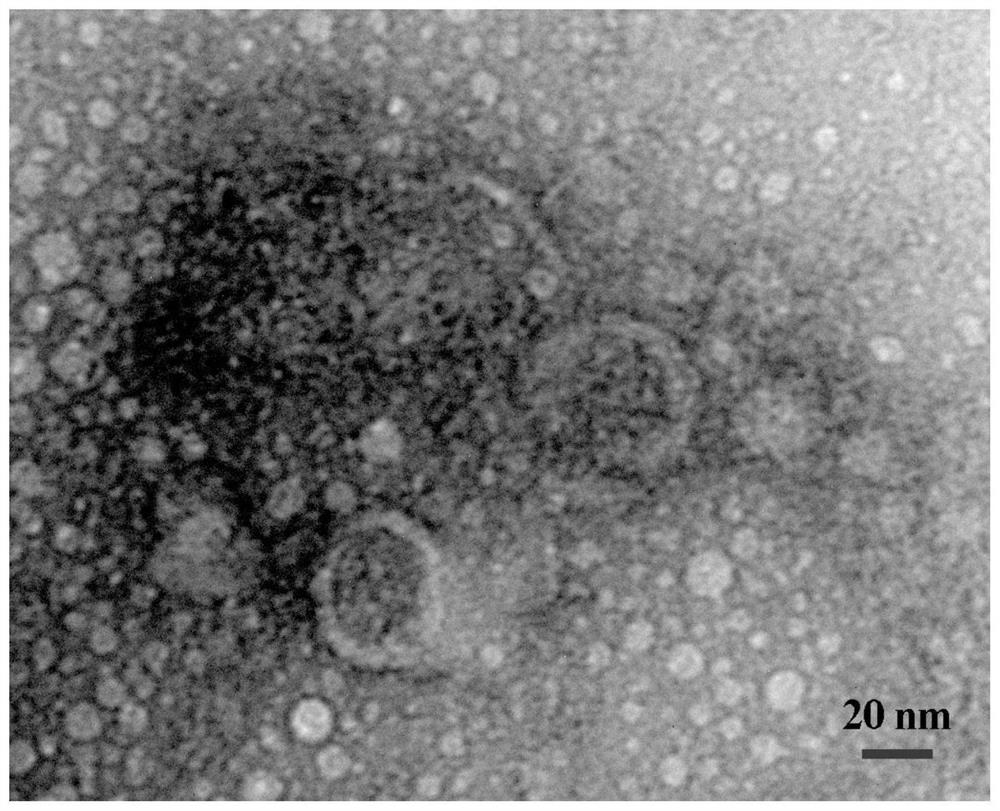
[0040] The extracted exosomes derived from adipose-derived mesenchymal stem cells, rabbit corneal epithelial cells and rabbit adipose-derived mesenchymal stem cells were subjected to next-generation sequencing (sequencing was completed by Guangzhou Huayinkang Medical Group Co., Ltd.). Then, the data was analyzed and screened based on the principle of the expression of the selected miRNA and the high expression of adipose-derived mesenchymal stem cells, and finally one miRNA was screened and a double-stranded miRNA mimic (ie: miRNA24-3p mimic) was synthesized for functional experiments; ,
[0041]The nucleotide sequence of the miRNA 24-3p mimic is: TGGCTCAGTTCAGCAGGAACAG (SEQ ID NO: 1);
[0042] miRNA 24-3p mimics:
[0043] Forward primer: UGGCUCAGUUCAGCAGGAACAG (SEQ ID NO: 2);
[0044] Reverse primer: CUGUUCCUGCUGAACUGAGCCA (SEQ ID NO: 3).
Embodiment 3
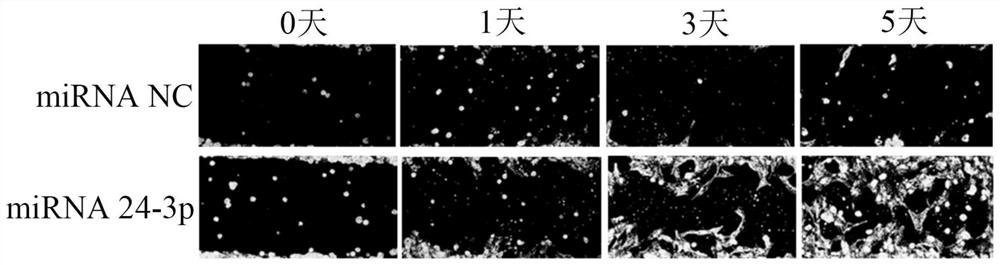
[0046] Follow Lipofectamine TM RNAiMAX transfection reagent (purchased from Invitrogen Company) instruction manual transfected rabbit corneal epithelial cells, namely: ① inoculation of 0.25-1 × 10 6 Put rabbit corneal epithelial cells (purchased from Beina Biotechnology) into 6-well plates and incubate for 4 hours; ②Take 9 μL of Lipofectamine TM Add RNAiMAX Transfection Reagent to 150 μl Culture medium (purchased from Thermo Fisher Scientific (China) Co., Ltd.) was mixed evenly to obtain mixed solution 1; Mix well in the culture medium to obtain mixed solution 2; ④ fully mix the mixed solution 1 obtained in step ② and the mixed solution 2 obtained in step ③ according to the ratio of 1:1 by volume, and incubate at room temperature for 5 minutes to obtain mixed solution 2. Solution 3; ⑤ Take 250 μL of the mixed solution 3 in step ④ and add it to the 6-well plate in step ①, then add 1 mL of complete medium in an incubator with 5% CO 2 Incubate at 37° C. for 1-3 days to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com