Biomarkers and agents for recurrent implant failure
A technology of biomarkers and medicaments, applied in the fields of biochemical equipment and methods, microbial determination/inspection, drug combination, etc., can solve the problems of lack of curative effect and treatment methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] sample
[0062] The overall condition of the patient was good, with no immune and chromosomal abnormalities, and no history of recurrent miscarriage. We analyzed a total of 84 patients with low progesterone levels and RIF from the Sixth Medical Center of the Chinese People's Liberation Army General Hospital, and all patients signed the informed consent. The characteristics of the patients are as follows: (1) repeated implantation failure (RIF, no clinical pregnancy was observed in three consecutive high-quality embryo transfers); (2) aged between 35 and 45 years old. This study was approved by the ethics committee of the medical center.
[0063] According to progesterone support therapy, patients were divided into two groups: experimental group and control group. The experimental group received low-dose human chorionic gonadotropin (HCG) on the basis of traditional progesterone therapy, and the control group maintained traditional progesterone therapy.
[0064] All s...
Embodiment 2
[0076] For the samples in Example 1, different luteal phase support schemes were adopted, and the patients were divided into a control group and an experimental group.
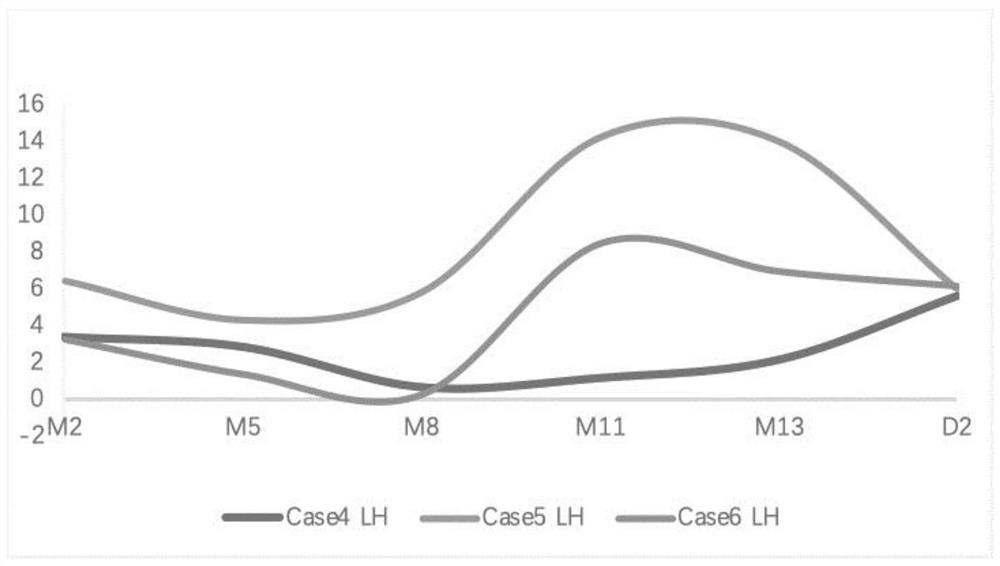
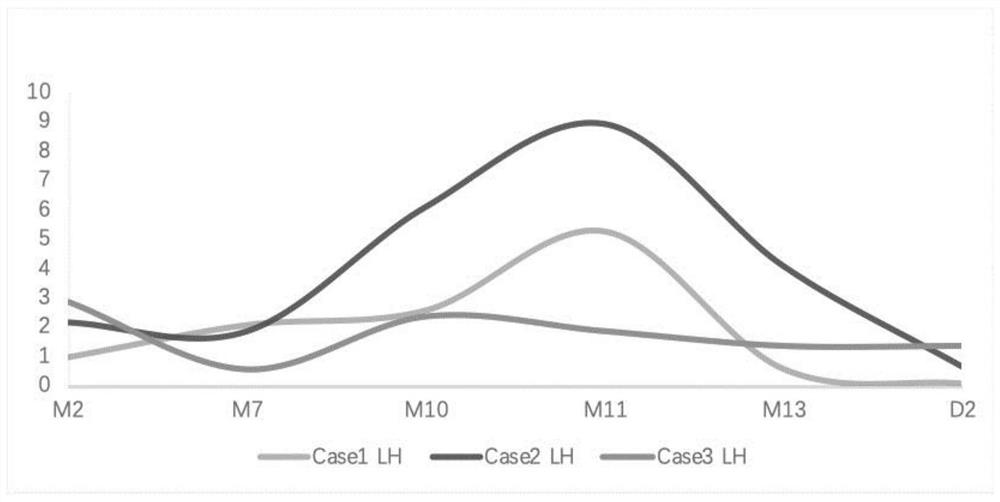
[0077] Experimental group: On the day of endometrial transition, the patients began to take femotone (yellow tablet, containing 2 mg estradiol, 10 mg dydrogesterone), progesterone soft capsules and prednisone (adrenocortical hormone). The dosage is as follows: femotone 1 tablet / time, 3 times / day, progesterone soft capsule 0.2g / time, 2 times / day, aspirin 25mg / time, 3 times / day, prednisone 5mg / time / day; ovulation Three types of hormones (E 2 , LH, P) The dose was determined according to the estrogen and progesterone levels on D2. On the next day after transplantation, the patient received 500 IU intramuscular injection of HCG every day, and other drugs and doses remained the same as before. On the seventh day after implantation, evaluate HCG, E 2 , LH,, P level, when the HCG level is higher than 50mIU / mL, the...
Embodiment 3
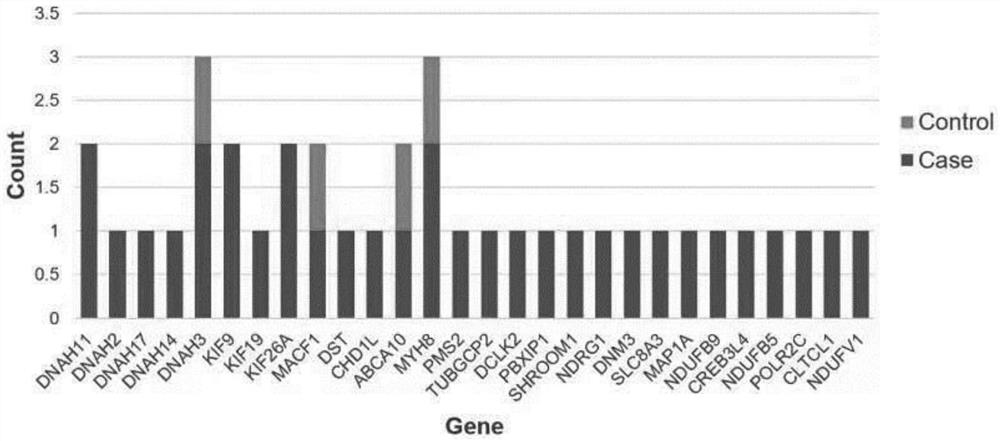
[0094] For the samples in Example 1, all samples were sequenced while being treated with the luteal phase support regimen, and peripheral blood was extracted from the patient to obtain genomic DNA, and all blood samples for sequencing were collected before the patient became pregnant.
[0095] DNA extraction, sequencing library construction
[0096] DNeasy tissue and blood kit was used for peripheral blood DNA extraction. In order to confirm the DNA degradation and contamination status, we chose Qubit DNA detection kit and used Qubit 3.0 fluorescent agent for 1% agarose gel electrophoresis test. The DNA extracted from the sample The quality and quantity status are shown in Table A. Samples with DNA content greater than 0.6ug were selected for library construction. Afterwards, the Agilent Human All-Exon V6 Kit was used to enrich DNA in the human exon region and generate a sequencing library according to the instructions. After the library is built, use Qubit 2.0 for prelimin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com