Synthesis method of novel fluorescent functional molecule glycoluril benzylamine carboxylic acid derivative
A technology of molecular glycoluril benzylamine carboxylic acid and glycoluril benzylamine carboxylic acid, which is applied in the field of synthesis of fluorescent functional small molecule compounds, can solve problems such as inconvenient operation, limitations, and poor water solubility of molecular tweezers, and achieve cost reduction and product yield High and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
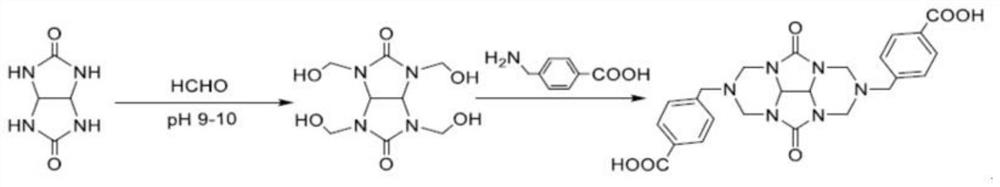
[0030] The synthetic method of Tetramethylol glycoluril, its step is as follows:
[0031] S1: Add 7.1g glycoluril, 8.9g paraformaldehyde, 25ml distilled water to the three-necked flask, and then add 1M Na 2 CO 3 Adjust the pH value to 9-10, raise the reaction temperature to 45°C, and react for 2 hours;
[0032] S2: After the reaction of S1 is completed, the reactant is cooled to room temperature, concentrated under reduced pressure to a viscous state; 40ml of methanol is added, and stirred for 4 hours by a magnetic stirrer, and solids continue to precipitate during the stirring process;
[0033] S3: The solid precipitated in S2 was suction-filtered, and the filter cake was washed with a small amount of methanol, and vacuum-dried to obtain 5.92 g of white solid, which was tetramethylol glycoluril, and the calculated yield was 45.2%;
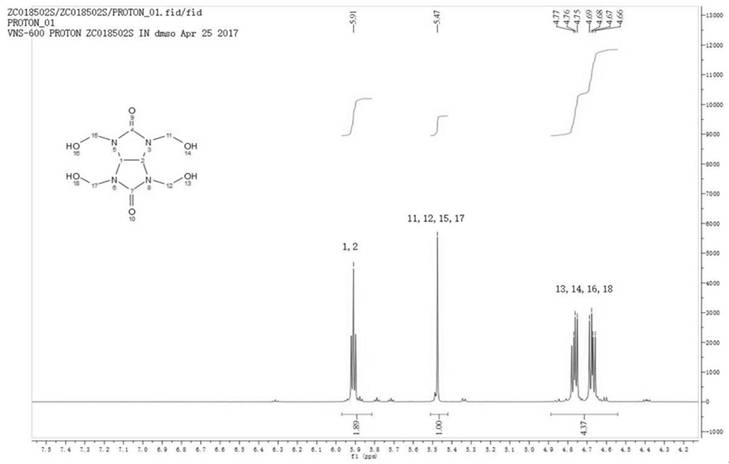
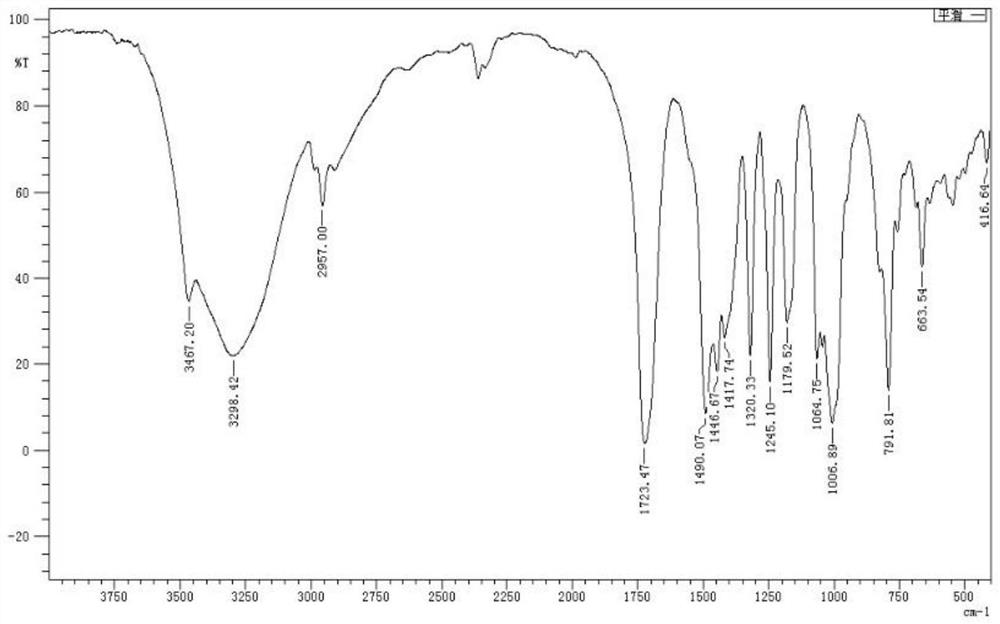
[0034] The infrared, NMR, carbon, and mass spectrometry data of tetramethylol glycoluril are:
[0035] IR(KBr)υ(cm-1)=3467,3298,2957,1723,1490...
Embodiment 2
[0039] The synthetic method of glycoluril benzylamine carboxylic acid derivative, its step is as follows:
[0040] S4: Take 1.31g of tetramethylol glycoluril prepared in Example 1, 1.63g of p-benzylaminobenzoic acid, and 20ml of distilled water were added to a 100ml three-necked flask, stirred with a magnetic stirrer, heated to 45°C, and obtained White suspension, add NaOH to adjust the pH to 8-9, the solution gradually becomes clear, continue heating to 950°C, react for 2.5h, stop heating, and cool to room temperature;
[0041] S5: Add HCl to the reaction solution at room temperature in S4 to adjust the pH to 5-6, and a white solid precipitates out. After filtration, take a white solid filter cake, wash the filter cake with methanol and water respectively, and vacuum dry to obtain 0.9033 g of a white solid, namely It is a glycoluril benzylamine carboxylic acid derivative with a yield of 36.7%.
[0042] The infrared, NMR, carbon, and mass spectrometry data of glycoluril benzy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com