Novel HPV therapeutic nucleic acid vaccine
A technology of HPV16-AVLC1 and HPV16-AVLS1, which is applied in the field of new HPV therapeutic nucleic acid vaccines, can solve the problems of weak immune response and limited therapeutic effect, and achieve the effect of efficiently inducing T cell immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 Design scheme, construction and preparation of nucleic acid sequence
[0069] 1. Obtainment of HPV16 / 18 E6, E7, L1 and L2 target fragments
[0070] Download the DNA sequence and protein sequence of E6 / E7 and L1 / L2 of HPV16 strain NC_001526.4 from GenBank;
[0071] The DNA sequence and protein sequence of E6 / E7 and L1 / L2 of HPV18 strain AY262282.1 were downloaded from GenBank.
[0072] 2. Sequence optimization
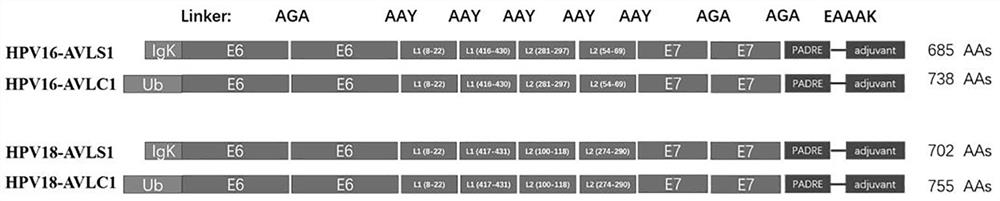
[0073] After splicing all the sequences according to the vaccine protein structure, HPV16-AVLS1 and HPV16-AVLC1 contain 685 and 738 amino acids, respectively, and HPV18-AVLS1 and HPV18-AVLC1 contain 702 and 755 amino acids, respectively, and then optimize the codons. These optimization methods Including but not limited to: human codon usage bias, moderate GC content, stable mRNA secondary structure, etc., eliminating repetitive sequences and cryptic splice sites and unwanted restriction enzyme sites, while preventing tRNA pools in cells of exhaustion...
Embodiment 2
[0077] Example 2 Recombinant plasmid digestion and expression identification
[0078] 1. Enzyme digestion identification
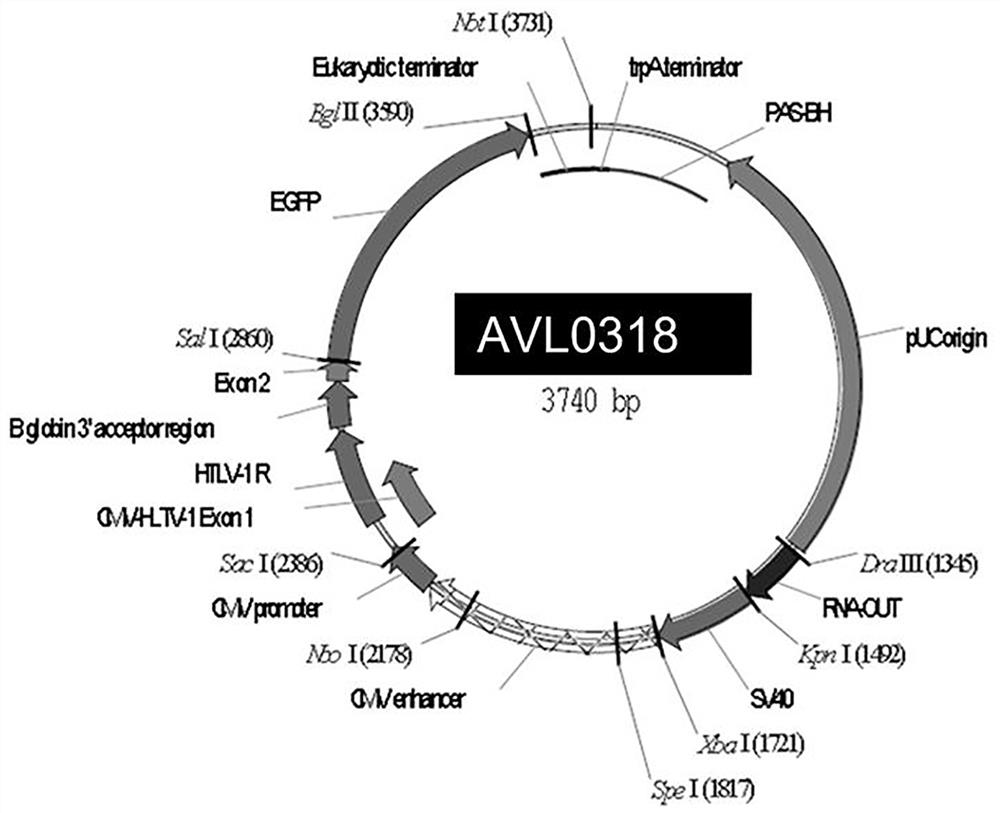
[0079] The plasmids of AVL0318-HPV16 / 18-AVLS1 / AVLC1 were amplified separately using Escherichia coli AVL-DH5α(SacB), and then purified using the endotoxin-free plasmid purification kit.
[0080] The specific operation method is as follows:
[0081] 1) Use AVL-DH5α(SacB) strain on LB medium with 6% sucrose, culture at 37°C for 24h.
[0082] 2) Use the plasmid extraction kit for plasmid extraction.
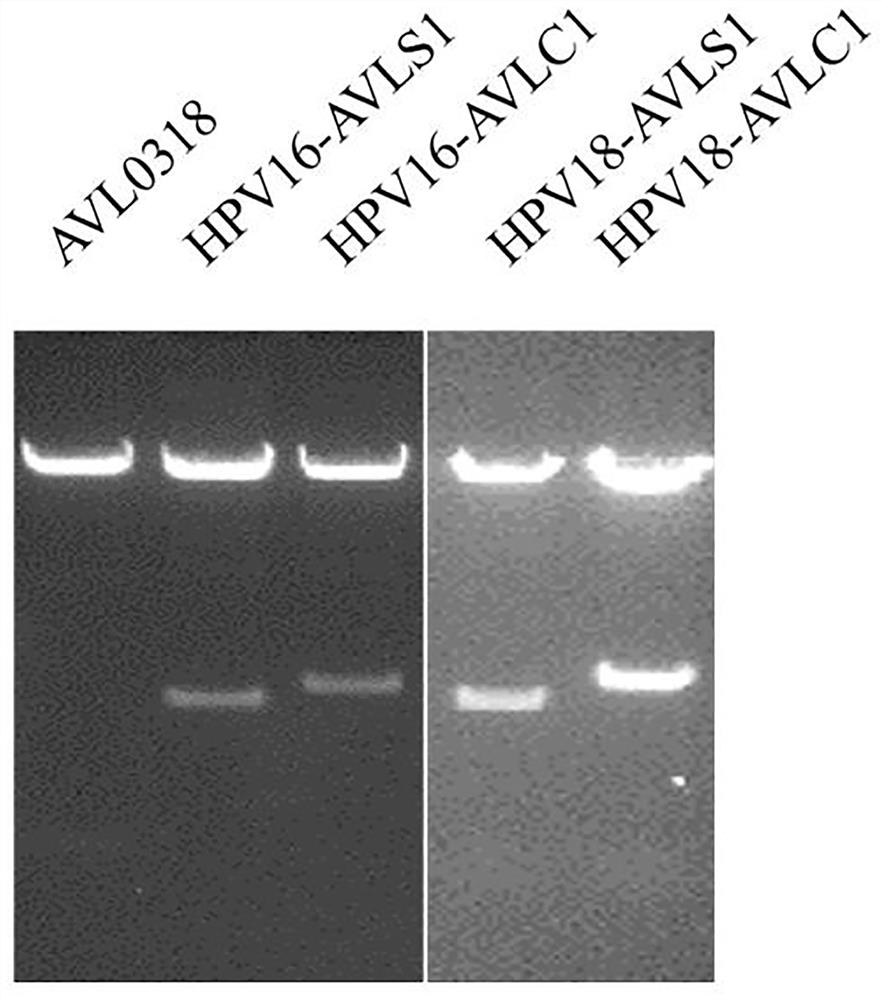
[0083] 3) Use HindIII and XhoI double digestion to verify the correctness of the plasmid, and sequence to verify the correct type of the sequence, such as image 3 shown.
[0084] 2. Expression Identification
[0085] Plasmid expression identification was performed using the HEK-293T cell line. HEK-293T cells were cultured in a 6-well plate, and 1.5 μg of the plasmid was transferred into the cells using lipofectamine2000. After 48 hours of transfection, th...
Embodiment 3
[0086] Example 3 Cellular Immunity Analysis
[0087] 3.1 Immunization, C57BL / 6J mice were subcutaneously administered 30 μg HPV16 DNA vaccines AVL0318-AVLS1 and AVL0318-AVLC1 (1:1) or 30 μg HPV18 DNA vaccines AVL0318-AVLS1 and AVL0318 in the pinna of each ear at 2-week intervals - AVLC1 (1:1) (plasmid dissolved in TE buffer, i.e. 15 μg (20 μL) / ear) immunized 3 times. Use 3-5 mice per group. The immune response was detected one week after the three immunizations. ELISPOT of mouse splenocytes was used to detect E6 and E7 specific cellular immune responses.
[0088] 3.2 ELISPOT detection
[0089] 3.2.1 Acquisition of splenocytes
[0090] Take about 500 μL of blood from the mice in a 1.5mL EP tube, let it stand at room temperature for about 1 hour, take the serum and store it at -80°C; the mice are killed by neck dislocation, soaked in alcohol for 5 minutes, then transferred to the ultra-clean bench, laparotomy, and the spleen is taken . The taken spleen was gently ground, d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com