Chiral bicyclic gamma-butyrolactone compound and application thereof
A butyrolactone and compound technology, applied in the field of chiral bicyclic γ-butyrolactone compounds, can solve the problems of complex synthesis steps, no absolute configuration of products and specific examples, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail below in combination with specific embodiments and with reference to the accompanying drawings. It should be understood that these descriptions are exemplary only, and are not intended to limit the scope of the present invention. Also, in the following description, descriptions of well-known structures and techniques are omitted to avoid unnecessarily obscuring the concept of the present invention.
[0033] The chiral diprimary amine catalyst structural formula of the present invention is:
[0034]
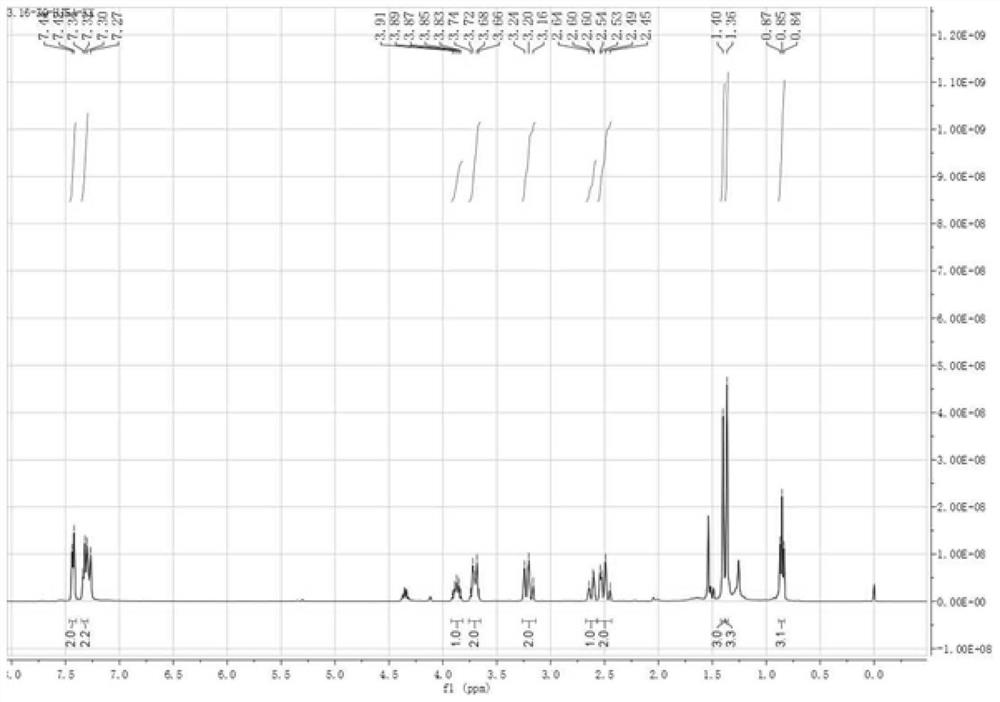
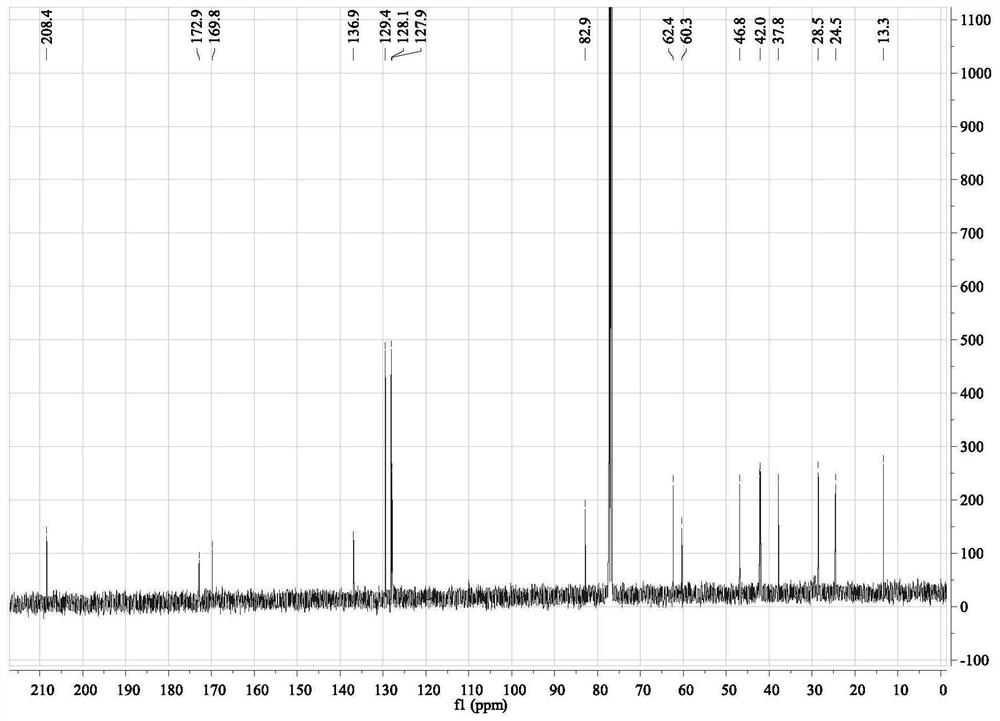
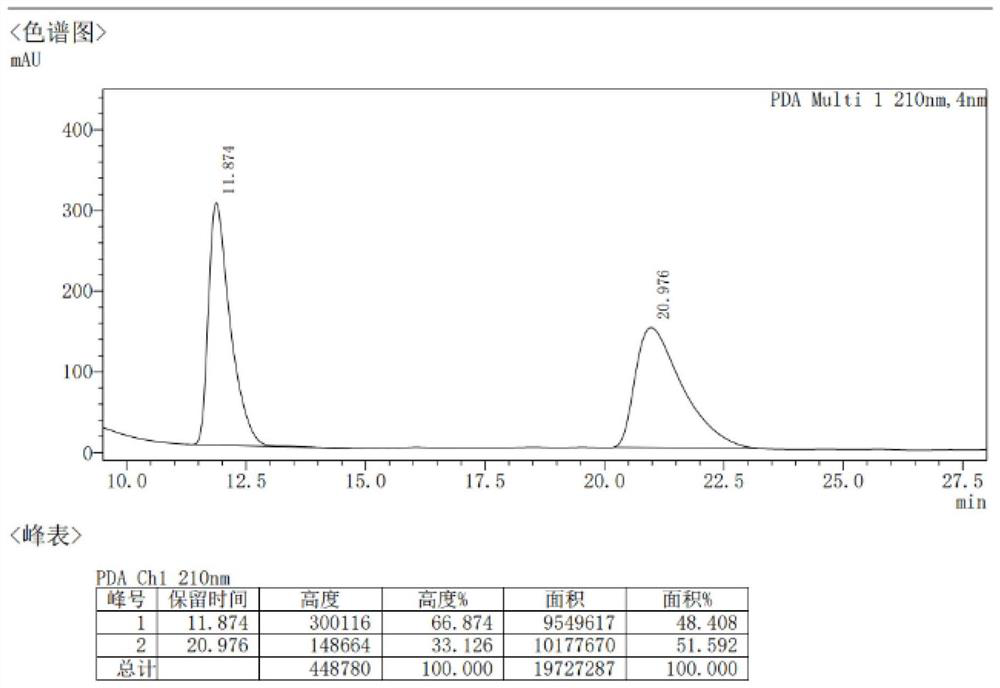
[0035] 1. Compound 3aa: take catalyst (1S,2S)-1,2-diphenylethylenediamine (0.05mmol) and N-Boc-L-Phg (0.05mmol) acidic additive and dissolve in solvent isopropanol (2.0mL ), pre-reacted for 15 minutes, added 1a (0.25mmol) and 2aa (0.5mmol) to the system, stirred at room temperature for 14 days, after the reaction was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com