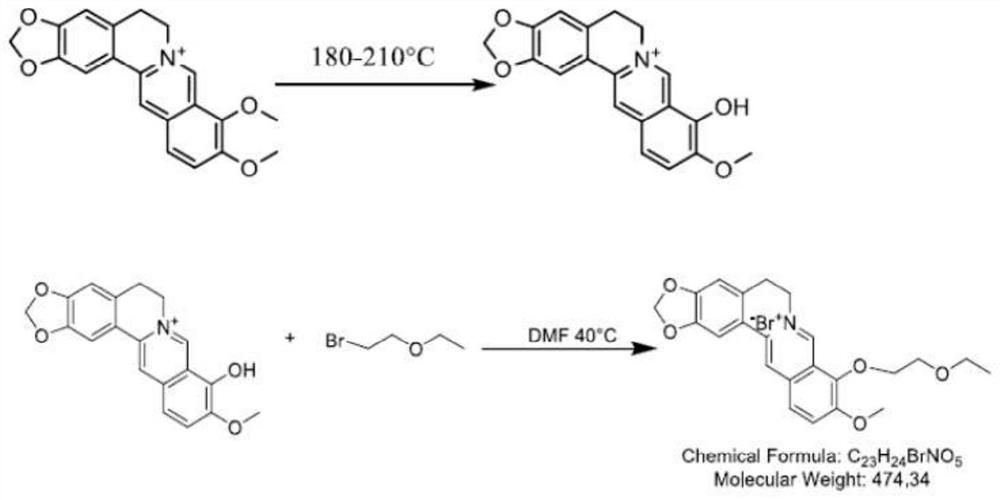
Synthesis method of 9-0-ethyl ether berberrubine and application of 9-0-ethyl ether berberrubine in preparation of antitumor drugs
A technology of berberine and bromoethyl ether, which is applied in the fields of drug synthesis and medical health, can solve the problems of poor solubility, low bioavailability, and poor druggability of berberine, and achieve anti-osteosarcoma, anti-colon cancer, Proliferation inhibiting, cost-effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19
[0052] Example 1 Preparation of 9-0-ethyl ether berberine
[0053] Weigh 2 g of berberine hydrochloride and place it in a 100 mL round bottom flask, add a stirrer bar, 50 Ml DMF and some zeolite to it, and react with microwave under 240 W for 20 minutes. After the reaction was completed and cooled, the solvent DMF was distilled off under reduced pressure. Purify by dry silica gel column chromatography, the eluent is V (dichloromethane): V (methanol) = 10: 1 for elution, and the solvent is evaporated under reduced pressure to obtain berbererythrine as a dark red solid.
[0054] Take berberine (12g, 37mmol) in a 250mL round-bottomed flask, use DMF (3mL) as a solvent, slowly add 2-bromoethyl ether (24mL, 100mmol) dropwise at 50°C, and react at 50°C for 24 hours . Extract with aqueous sodium bicarbonate (20mL×3), combine the organic phases, extract the organic phases with saturated brine (30mL×3), combine the organic phases, dry over anhydrous sodium sulfate, filter, and concent...
Embodiment 1
[0057] Target compound (embodiment 1) structure identification
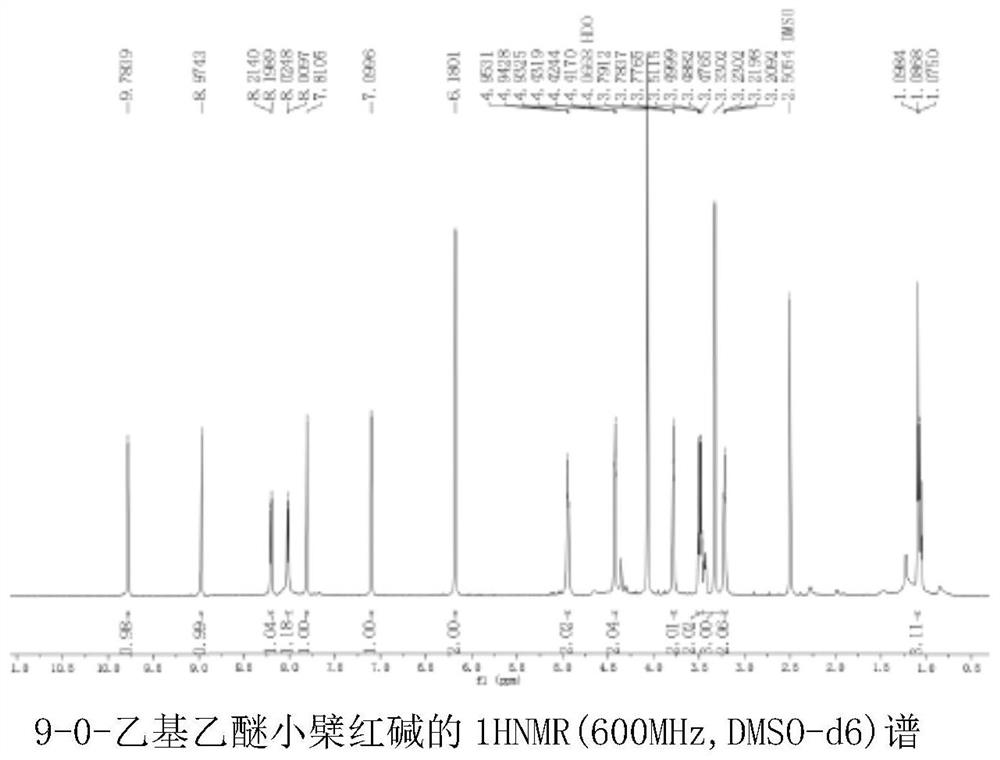
[0058] 1H-NMR (600MHz, DMSO-d6) δ9.78(s, 1H), 8.97(s, 1H), 8.21(d, J=9.1 Hz, 1H), 8.02(d, J=9.1Hz, 1H), 7.81(s,1H),7.10(s,1H),6.18(s,2H),4.94(t,J=6.2Hz,2H),4.42(t,J=4.5Hz,2H),3.78(t,J =4.4 Hz, 2H), 3.47(dq, J=27.4, 6.7Hz, 2H), 3.33(s, 2H), 3.22(t, J=6.3 Hz, 2H), 1.07(dt, J=18.0, 7.0Hz ,4H).
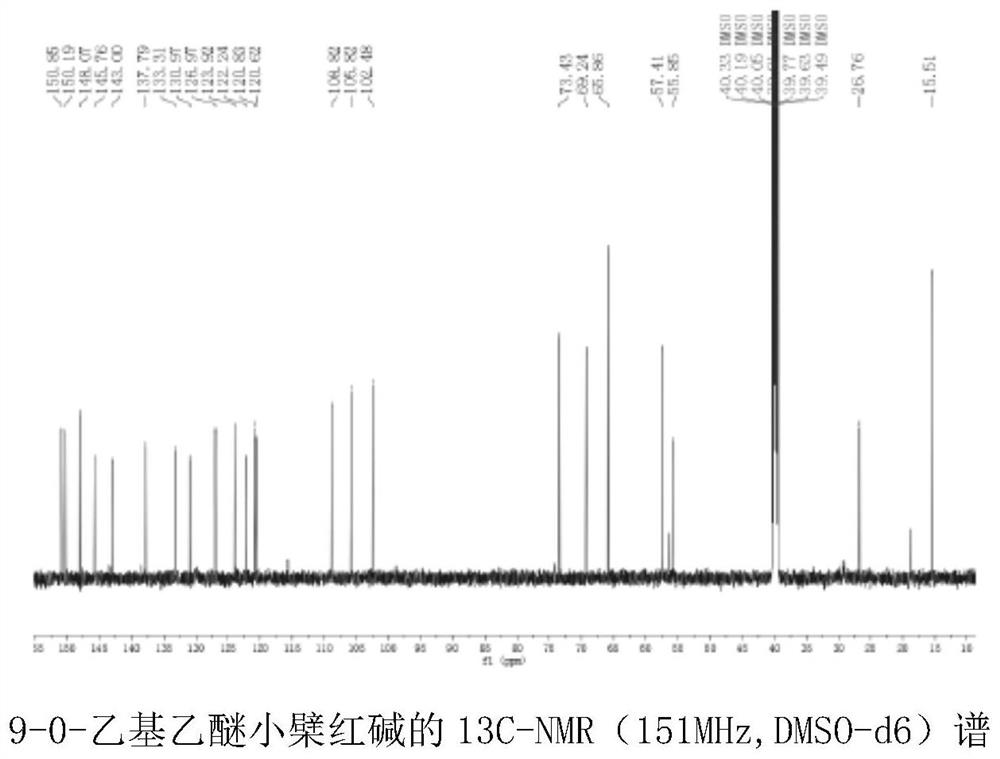
[0059] 13C NMR(151MHz,DMSO)δ150.85,150.19,148.07,145.76,143.42,143.00,137.79,133.31,130.97,126.97,123.92,122.24,120.83,120.62,115.67, 108.82,105.82,102.48,98.80,73.43,69.24,65.86 , 57.41, 56.39, 55.85, 40.44, 40.33, 40.19, 40.05, 39.91, 39.77, 39.63, 39.49, 29.37, 26.76, 18.94, 15.51.
[0060] Based on the above information, it is determined that the structure of the compound is as shown in formula I, wherein n=1,9-0-ethyl ether berberine nuclear magnetic resonance spectrum is as follows figure 2 shown.
Embodiment 3
[0061] Efficacy test 1 of embodiment 3 product of the present invention
[0062] Study on the effect of 9-0-ethyl ether berberine on the growth of 143B human osteosarcoma cell subcutaneous xenograft 1. Experimental materials Experimental animals: BALB / c-nu nude mice, male, weighing 18±2g, 48. Provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[0063] Main drugs: doxorubicin hydrochloride, berberine, 9-0-ethyl ether berberine (prepared in Example 1)
[0064] 2. Experimental method
[0065] (1) Construction of animal model of osteosarcoma: The animal model of ectopic osteosarcoma was established by subcutaneously injecting 143B human osteosarcoma cells in the back of nude mice. After the 143B human osteosarcoma cells were collected, a cell suspension of 5×105 cells / mL was prepared, and 100 μL of the cell suspension was subcutaneously injected into the back of the neck of each nude mouse.
[0066](2) Experimental grouping: 48 male nude mice were random...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com