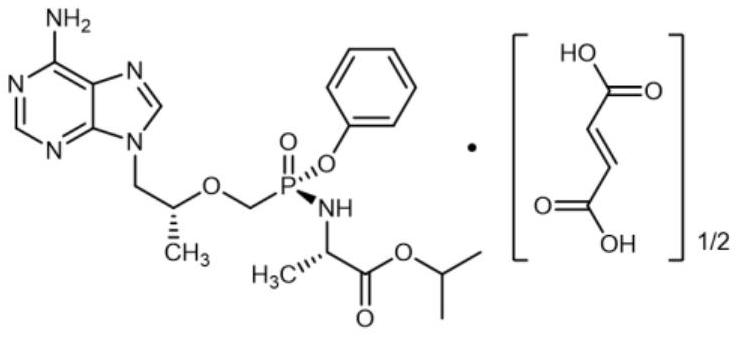
Preparation method of propofol tenofovir lactose impurity
A technology of tenofovir and propofol, applied in the field of pharmaceutical impurity preparation, can solve the problems of no literature report TAF lactose-binding impurity preparation method and the like, and achieve the effects of simple synthesis method, improved quality and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Tenofovir alafenamide (30g) and dimethyl sulfoxide (315mL, moisture content: 0.05%) were added to a 500mL reaction flask, stirred into lactose (86g), and reacted at a temperature of 90-95°C for 35 hours. The reaction liquid was distilled off under reduced pressure to remove dimethyl sulfoxide to obtain a crude product, which was purified with a preparative column, and the preparation conditions were as follows:
[0031] Octadecyl bonded silica gel was used as the stationary phase, mobile phase A: ammonium bicarbonate solution, and mobile phase B: acetonitrile. The gradient elution conditions are as follows:
[0032] Time(min) mobile phase A mobile phase B 0 70% 30% 21 50% 50% 21.5 5% 95% 26 5% 95% 26.5 90% 10% 30.5 90% 10%
[0033] The obtained preparation solution was freeze-dried to obtain TAF lactose-binding impurities with an HPLC purity of 97.2%.
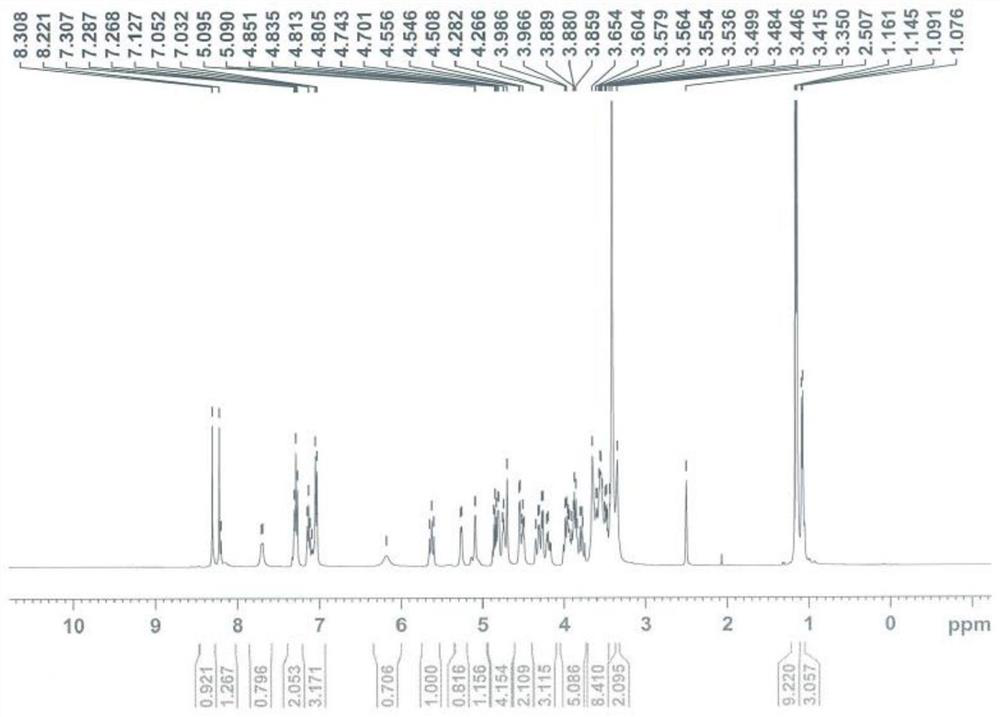
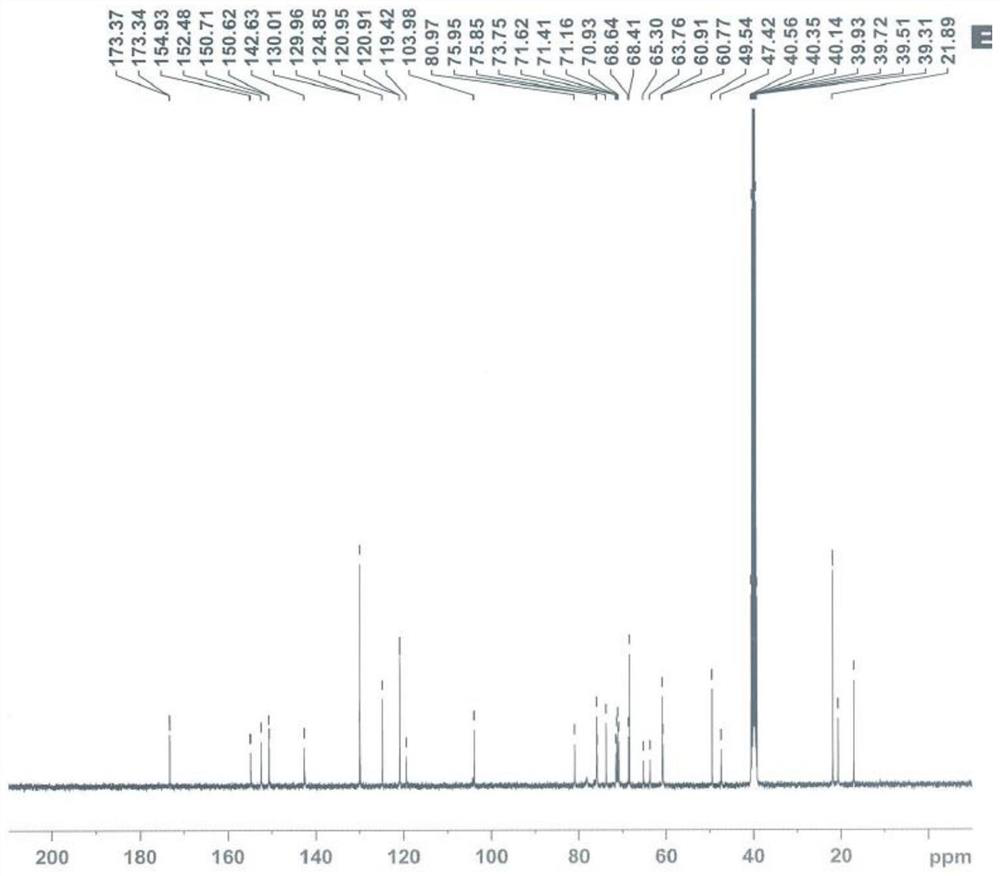
[0034] Data Analysis of TAF Lactose Binding Impurity Structure...
Embodiment 2
[0039] Add tenofovir alafenamide (20g) and N,N-dimethylformamide (200mL, water content: 0.02%) into the reaction flask, stir and add lactose (57g), and react at a temperature of 90-95°C for 40 hours , the reaction solution was distilled off under reduced pressure to remove N,N-dimethylformamide to obtain a crude product.
[0040] The crude product was purified by a preparative column, the preparation conditions were the same as in Example 1, and the obtained preparation solution was freeze-dried to obtain TAF lactose-bound impurities with an HPLC purity of 95.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com