Refining method of ophthalmic protein medicine
A buffer solution and cation exchange technology, which is applied in the preparation method of peptides, animal/human proteins, drug combinations, etc., can solve the problems that have not yet revealed the specific conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0083]
[0084] (1) Cation exchange chromatography
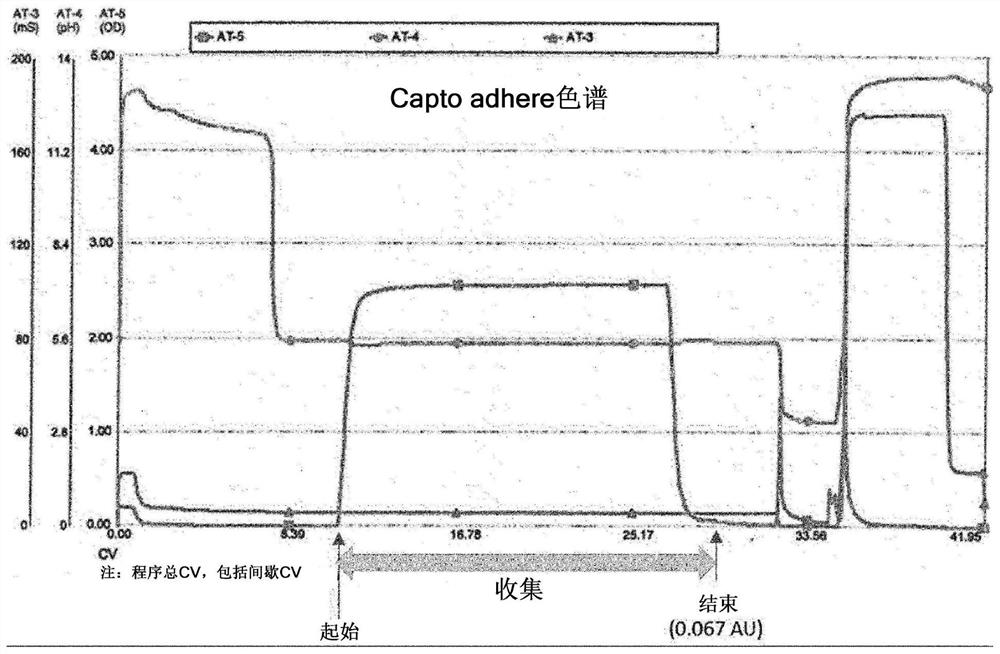
[0085] Cation exchange chromatography was performed on aflibercept sample solutions collected by protein A affinity chromatography or from aflibercept sample solutions collected by performing protein A affinity chromatography and multimodal chromatography together to purify only the Aflibercept substance of electric point value.
[0086] Examples of charged functional groups with cation exchange resins used in the present invention can be carboxymethyl (CM), sulfoethyl (SE), sulfopropyl (SP), phosphate (P), etc., cation exchange resins Examples of carriers can be polystyrene, polystyrene / divinylbenzene, cellulose, dextran, agarose, and Toyopearl. Examples of cation exchangers used in the present invention have carboxymethyl groups (CM) in particular.
[0087] Any buffer solution that may have buffering capacity can be used as the buffer solution used in the cation exchange chromatography process of the present invention,...
example 2
[0104]
[0105] (1) Anion exchange chromatography
[0106] Anion-exchange chromatography was performed on aflibercept sample solutions collected by protein A affinity chromatography or from protein A affinity chromatography and multimodal chromatography performed together to purify only those with a specific range Aflibercept substance of electric point value.
[0107] Examples of charged functional groups with anion exchange resins used in the present invention may be amines or amino groups, more preferably primary, secondary, tertiary or quaternary amines, and most preferably tertiary or quaternary amines, the anion exchange resins Examples of carriers may be polystyrene, polystyrene / divinylbenzene, cellulose, dextran, agarose, and Toyopearl. Examples of anion exchangers used in the present invention have in particular diethylaminoethyl (DEAE) ligands and quaternary ammonium (Q) ligands.
[0108] Any buffer solution that may have buffering capacity can be used as the buf...
example 3
[0125]
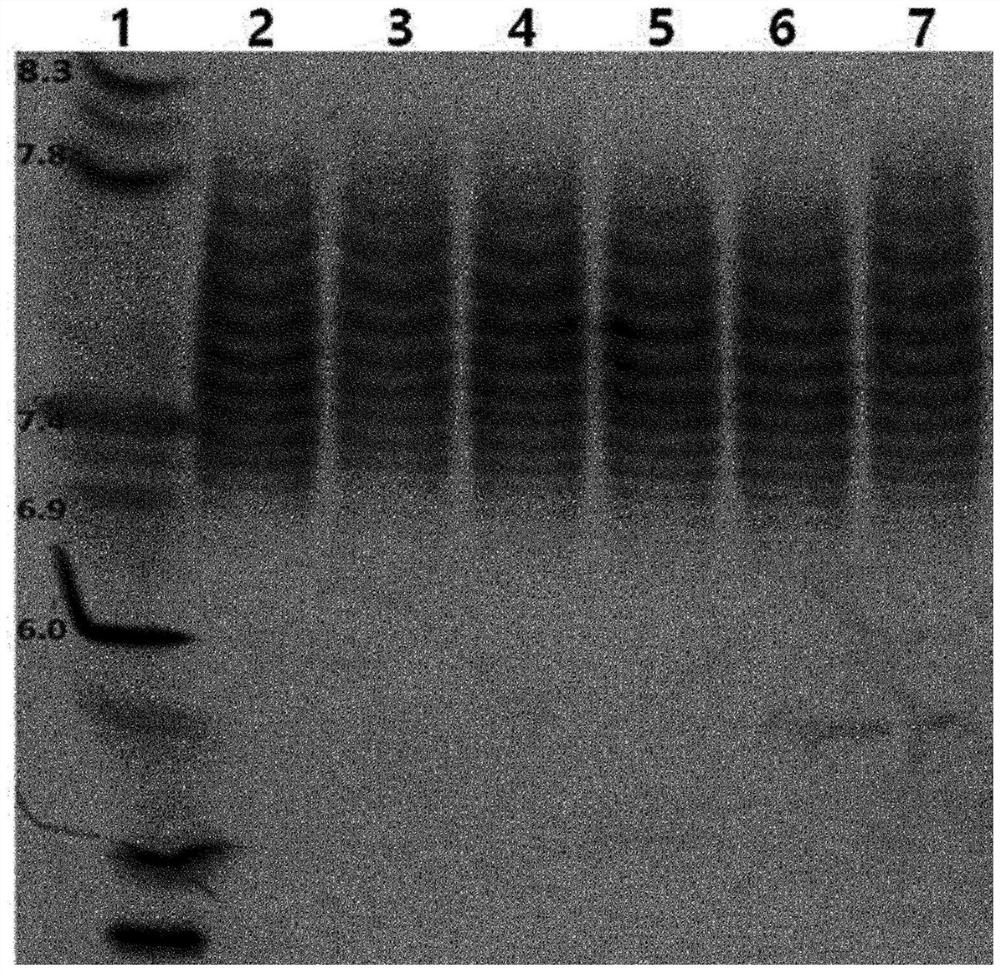
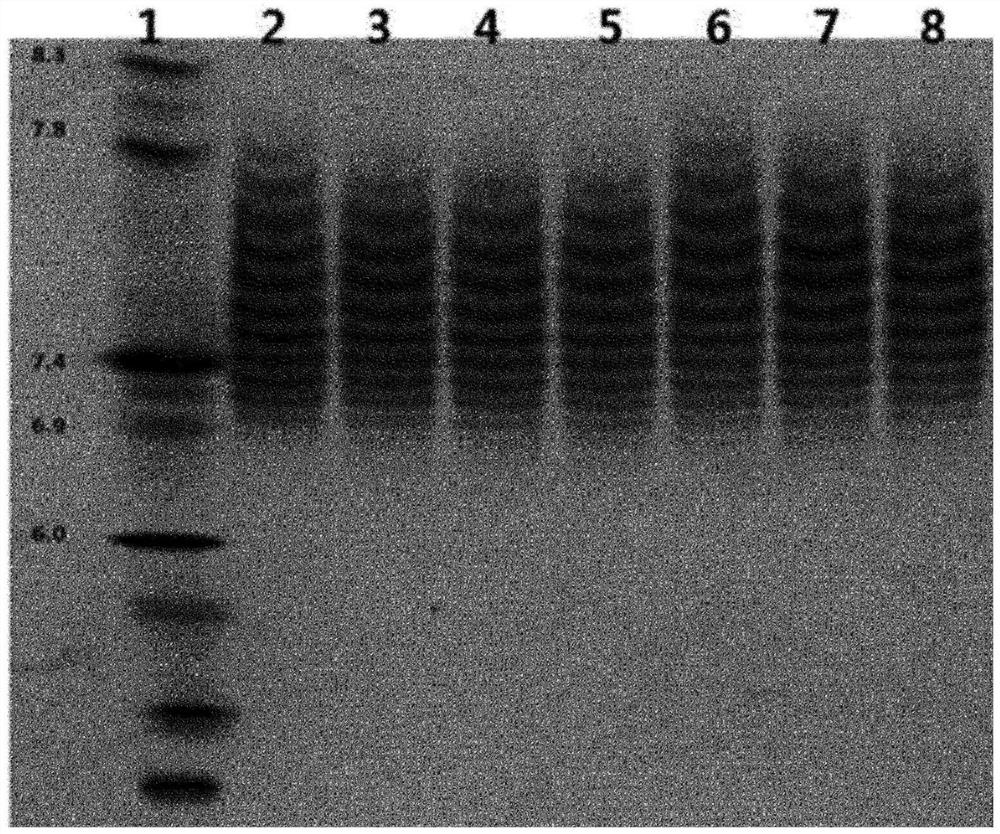
[0126] Aflibercept sample solutions collected by protein A affinity chromatography or aflibercept sample solutions from protein A affinity chromatography and multimodal chromatography performed together were subjected to combined application of cation exchange chromatography and anion exchange chromatography to purify Only aflibercept substances with isoelectric point values within a specific range.
[0127] That is, according to the method of Example 1 and Example 2, the chromatography with CM-Sepharose FF resin as the cation exchange resin is first performed, and then the chromatography with the Q-Sepharose FF resin as the anion exchange resin is performed, or the chromatography with the Q-Sepharose FF resin is first performed Chromatography as anion-exchange resin followed by chromatography with CM-Sepharose FF resin as cation-exchange resin allows for easier purification of chromatographic substances having only a specific range (pH 6.0-8.3) than when performin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com