Application of vitamin D analogue for treating or preventing viral hepatitis
A technology for viral hepatitis and derivatives, applied in antiviral agents, drug combinations, organic active ingredients, etc., to achieve excellent clinical safety, excellent pharmacokinetic properties, and good druggability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
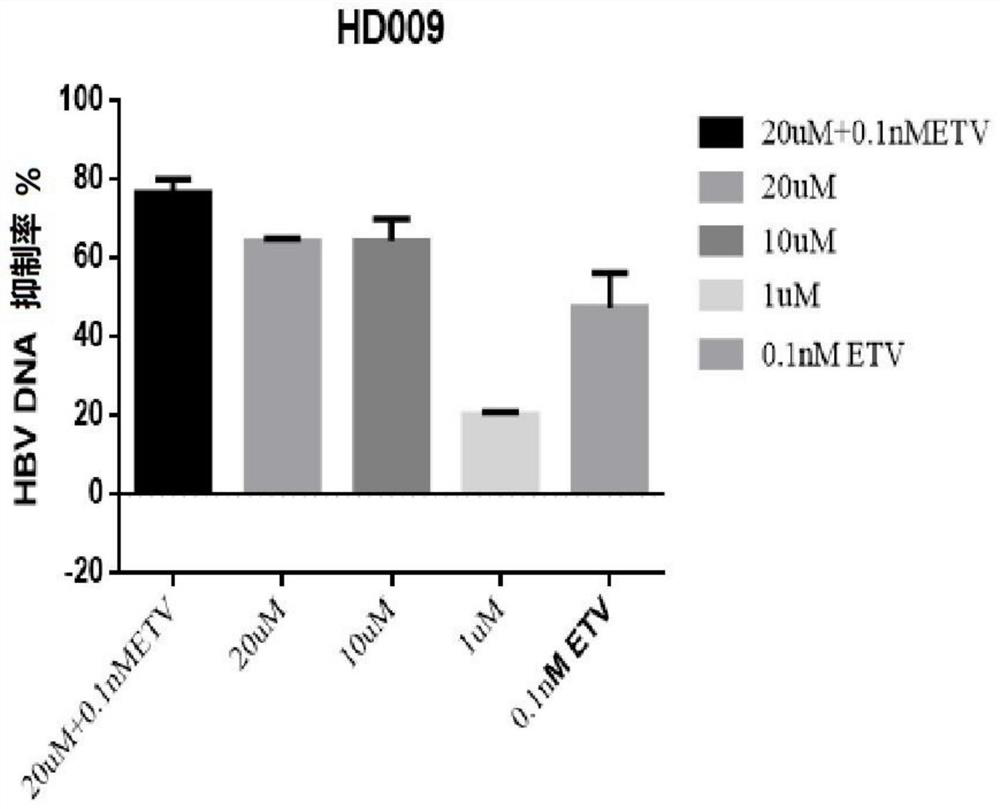
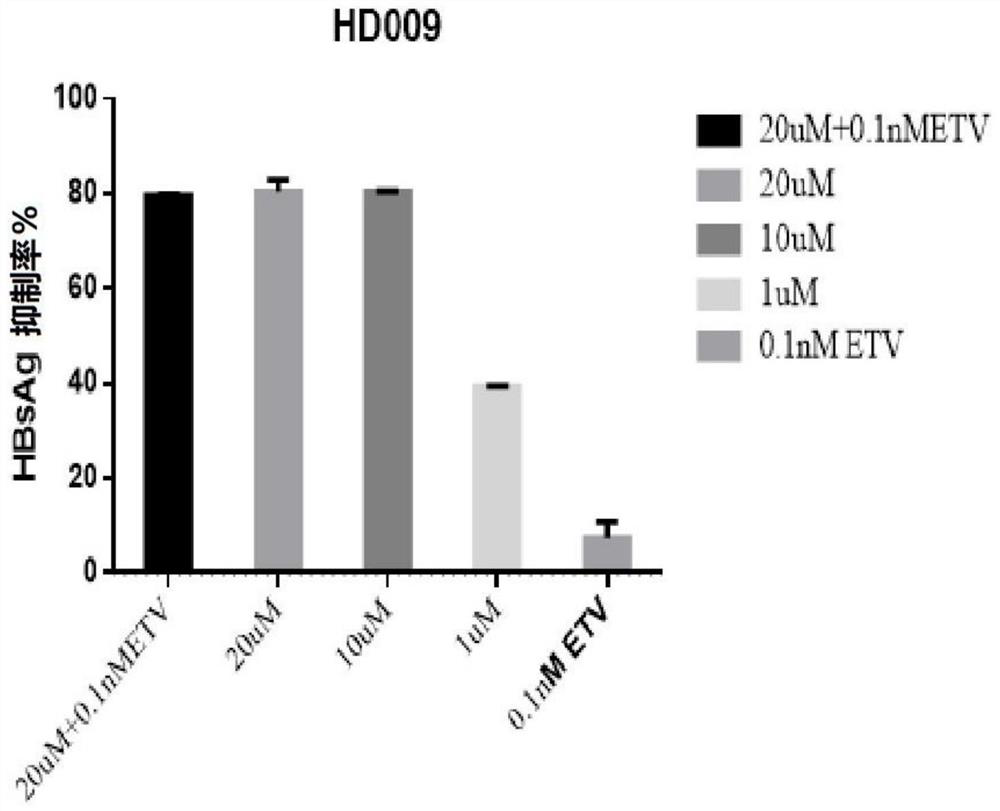
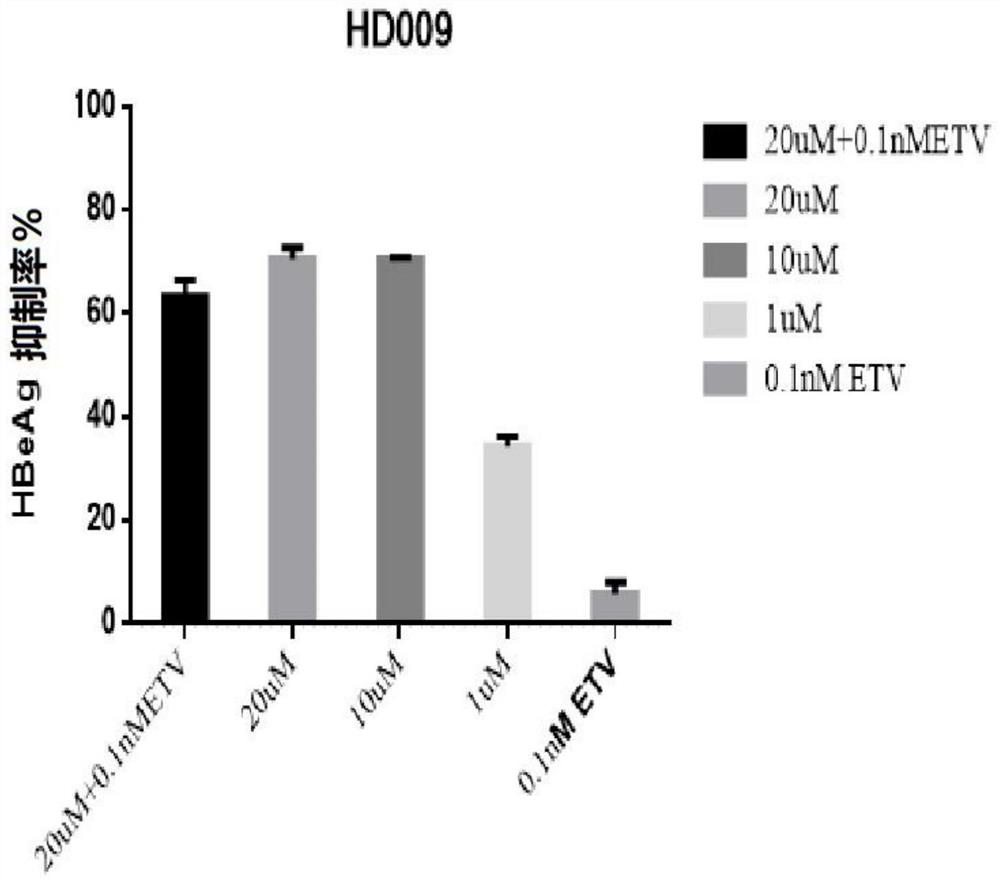
[0109] Example 1 - Application of HepG2-NTCP cells to evaluate the in vitro anti-HBV activity of paricalcitol (HD009, compound 9), alfacalcitol (HD125, compound 18) and calcifediol (HD135, compound 3)
[0110] Compound preparation method is as follows:
[0111] Take the preparation of 20mM concentration as an example, the volume of solvent DMSO (μl) = sample mass (mg) × purity ÷ molecular weight ÷ 20 × 10 6
[0112] Control compounds include ETV (Entecavir) (batch number: P1214012; 99.0% purity), purchased from Shanghai Titan Technology Co., Ltd.; HD131 (vitamin D2), HD132 (vitamin D3), HD134 (docalcitol), purchased from Shanghai Tao Su Biochemical Technology Co., Ltd. The concentration of the stock solutions of the above control compounds was 20 mM and stored at -20°C.
[0113] Table 1. Major Reagents and Cell Viruses
[0114]
[0115] Experimental program
[0116] Plating Cells and Compound Treatment
[0117] On day 0, HepG2-NTCP was plated into 48-well plates (7....
Embodiment 2-A
[0153] Embodiment 2-AAV HBV mouse model experiment
[0154]Administration was carried out according to the following table 7, and the content of HBV DNA, HBeAg and HBsAg in the cell culture supernatant was detected with reference to the same method as above, and the results are shown in Table 8:
[0155] Table 7
[0156] way of administration time blank 0, once a day 7 days HD125 2ug / kg, once a day 7 days
[0157] Table 8
[0158] HD125 Log HBV DNA Log HBsAg Log HBeAg 0day 6.56 3.41 2.66 7day 4.61 2.96 2.61 Variation -1.95 -0.45 -0.05
[0159] Animal experiment results also show that HD125 (alfacalcidol) can effectively reduce HBV DNA, HBsAg and HBeAg, showing good antiviral effect in vivo.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com