Metal organic complex containing benzimidazole skeleton as well as preparation method and application of metal organic complex
A metal-organic and benzimidazole technology is applied in the field of metal-organic complexes containing benzimidazole skeletons and the preparation thereof, and can solve the problems that the research is limited to the synthesis stage and the technology has not been further studied.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The invention provides a method for preparing the above-mentioned organometallic complex containing a benzimidazole skeleton, comprising the following steps:
[0055] The organic ligand having the structure shown in formula I, the transition metal compound and the organic solvent are mixed and reacted to obtain a metal-organic complex containing a benzimidazole skeleton.
[0056] In the present invention, the preparation method of the organic ligand having the structure shown in formula I preferably comprises the following steps:
[0057] Under the action of a catalyst and a base, the benzimidazole compound having the structure shown in formula A and the amino compound having the structure shown in formula B undergo carbon-nitrogen coupling reaction to obtain the organic ligand having the structure shown in formula I.
[0058] R 3 -NH 2 Formula B;
[0059] In Formula A and Formula B, R 1 is hydrogen, methyl or chlorine;
[0060] R 2 It is C1~C3 alkyl;
[0061] ...
Embodiment 1
[0073] The organic ligand and the transition metal compound were dissolved in methanol respectively, and then the methanol solution of the transition metal compound was added to the refluxing methanol solution of the organic ligand, and the mixed solution was continued to reflux for 4 hours, then cooled to room temperature, and the blue solid precipitate was filtered out That is the crude product. The crude product was added into DMF (dissolved completely), and then slowly diffused into the DMF solution by diethyl ether to obtain the corresponding single crystal pure product. This method yields Co-1 and Co-2.
[0074] The preparation process of Co-1 is shown in formula (1), and the preparation process of Co-2 is shown in formula (2).
[0075]
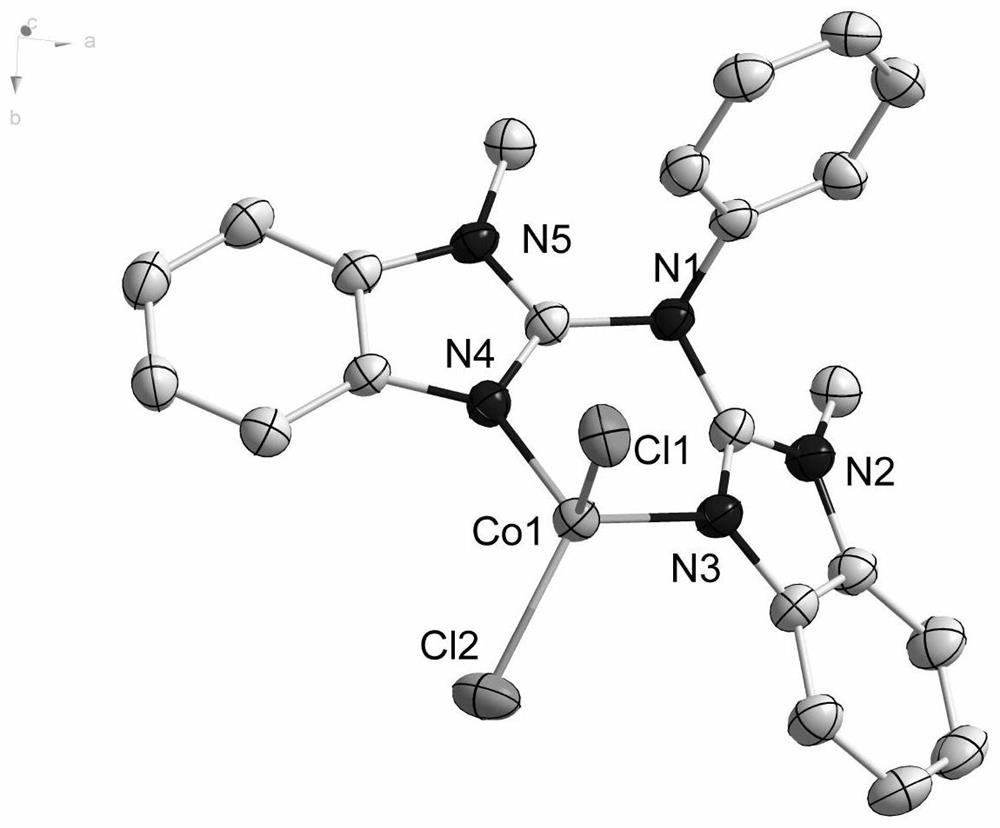
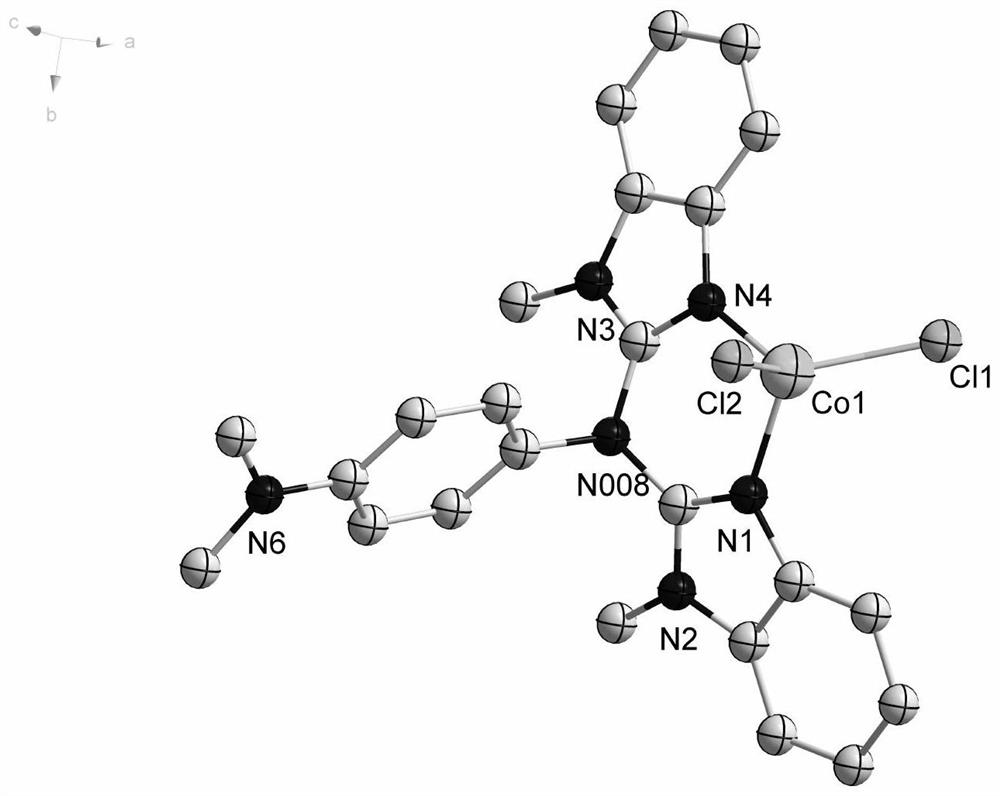
[0076] Among them, the single crystal structure of Co-1 is shown in figure 1 Shown; the single crystal structure of Co-2 is shown in figure 2 shown.
Embodiment 2
[0078] The ratio of the organic ligand to the transition metal compound is 2:1, dissolved in methanol, then the mixed solution is refluxed for 4 hours and then cooled to room temperature, the yellow solid precipitated by filtration is the crude product, and then the mixed solvent of diethyl ether and ethyl acetate Pure product was obtained after rinsing 3 times. This method yields Ir-1 and Ir-2.
[0079] The preparation process of Ir-1 is shown in formula (3), and the preparation process of Ir-2 is shown in formula (4).
[0080]
[0081] Among them, the nuclear magnetic data of Ir-1 is:
[0082] 1 H NMR (500MHz, DMSO-d 6 )δ7.86(d, J=8.3Hz, 2H), 7.66(d, J=8.3Hz, 2H), 7.53(t, J=7.6Hz, 2H), 7.47(t, J=7.7Hz, 4H) ,7.24(t,J=7.4Hz,1H),7.03(d,J=7.8Hz,2H),4.01(s,6H),1.39(s,15H). 13 C NMR (126MHz, DMSO-d 6 )δ145.1, 142.3, 137.1, 134.4, 131.3, 125.3, 124.7, 124.4, 119.5, 114.6, 113.2, 88.0, 32.4, 9.2.
[0083] The nuclear magnetic data of Ir-2 is:
[0084] 1 H NMR (500MHz, DM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com