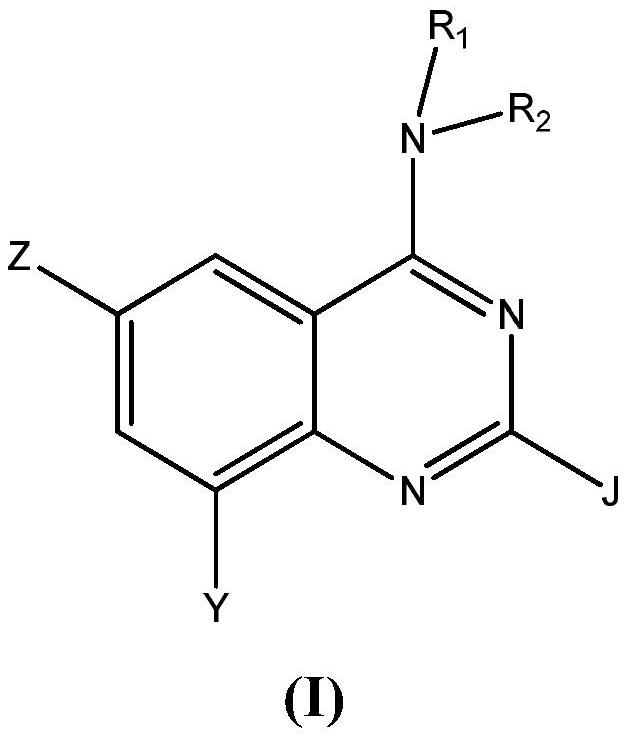
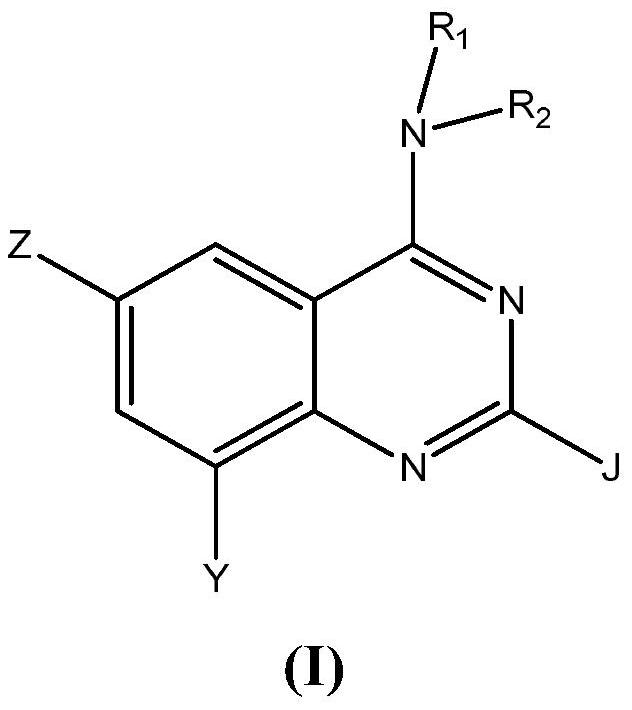
Aminoquinazoline derivatives as P2X3 inhibitors
An alkyl and aryl technology, applied in the direction of compounds containing elements of Group 3/13 of the periodic table, pharmaceutical combinations, medical preparations containing active ingredients, etc., can solve the problem of aminoquinazoline derivative compounds that are not described or suggested And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0442] Inhalable preparations include inhalable powders, propellant-containing metered dose aerosols or propellant-free inhalable preparations.
[0443] For administration as dry powder, single or multidose inhalers known from the prior art can be used. In this case the powder may be filled in gelatin, plastic or other capsules, cartridges or blister packs or in a depot.
[0444] Diluents or carriers that are chemically inert to the compounds of the invention, such as lactose or any other suitable additive for improving the respirable fraction, may be added to the powdered compounds of the invention.
[0445] Inhalation aerosols comprising a propellant gas such as a hydrofluoroalkane may contain a compound of the invention in solution or dispersion. Propellant driven formulations may also contain other ingredients such as co-solvents, stabilizers or optional other excipients.
[0446] The propellant-free inhalable formulations containing the compounds of the invention may be...
Embodiment 1
[0588] 6-(4-fluorophenyl)-8-methoxy-N-(1-(3-methyl-1,2,4- Oxadiazol-5-yl)ethyl)quinazolin-4-amine
[0589] Preparation of intermediate 1,2-amino-5-bromo-3-methoxybenzoic acid hydrobromide
[0590]
[0591] A solution of bromine (6.0 g, 1.9 mL, 37.70 mmol) in chloroform (15 mL) was added dropwise to 2-amino-3-methoxybenzoic acid (6.0 g, 35.90 mmol) in chloroform ( 180mL) in suspension. The reaction was stirred for another 5 hours and allowed to warm slowly to room temperature. The solvent was removed in vacuo and the residue was triturated with ether. The reaction was filtered to give the title compound (11.3 g, 96%) as a beige solid.
[0592] LCMS (Method 4): [MH+] = 247 at 4.07 min.
[0593] Preparation of intermediate 2 6-bromo-8-methoxyquinazolin-4-ol
[0594]
[0595] A solution of 2-amino-5-bromo-3-methoxybenzoic acid hydrobromide (Intermediate 1) (10.0 g, 30.60 mmol) in formamide (40 mL) was heated to 165 °C for 18 hours. After returning to room temperature...
Embodiment 114
[0646] N-(((1r,4r)-4-aminocyclohexyl)methyl)-6-(4-fluorophenyl)-8-methoxyquinazolin-4-amine
[0647]
[0648] Step 1: Benzyl ((1r,4r)-4-(((6-(4-fluorophenyl)-8-methoxyquinazolin-4-yl)amino)methyl)cyclohexyl)carbamate preparation of
[0649]
[0650] To a solution of 6-(4-fluorophenyl)-8-methoxyquinazolin-4-ol (Intermediate 3) (80mg, 0.30mmol) in N,N-dimethylformamide (2mL) (Benzotriazol-1-yloxy)tripyrrolidinophosphorus Hexafluorophosphate (169 mg, 0.32 mmol) and diisopropylethylamine (0.32 mL, 1.85 mmol). The resulting mixture was heated to 40 °C and stirred for 20 min, then benzyl ((1r,4r)-4-(aminomethyl)cyclohexyl)carbamate (93 mg, 0.36 mmol) was added and heating was maintained at 40 °C for 18 min. Hour. After returning to room temperature, the mixture was diluted with ethyl acetate (50 mL) and water (20 mL). The organic phase was washed with brine (2 x 20 mL), passed through a hydrophobic frit and the solvent was removed in vacuo. The residue was used directly i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com