Preparation method and application of pazufloxacin iridium complex
A technology for pazufloxacin and iridium complexes, applied in the field of preparation of pazufloxacin iridium complexes, achieving good spectral properties and cationic properties, low cytotoxicity, and the effect of inhibiting infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
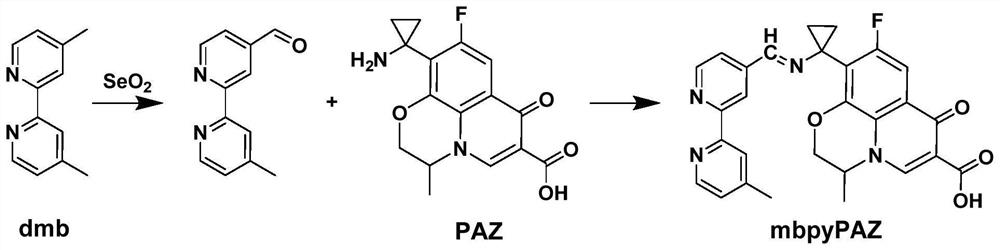
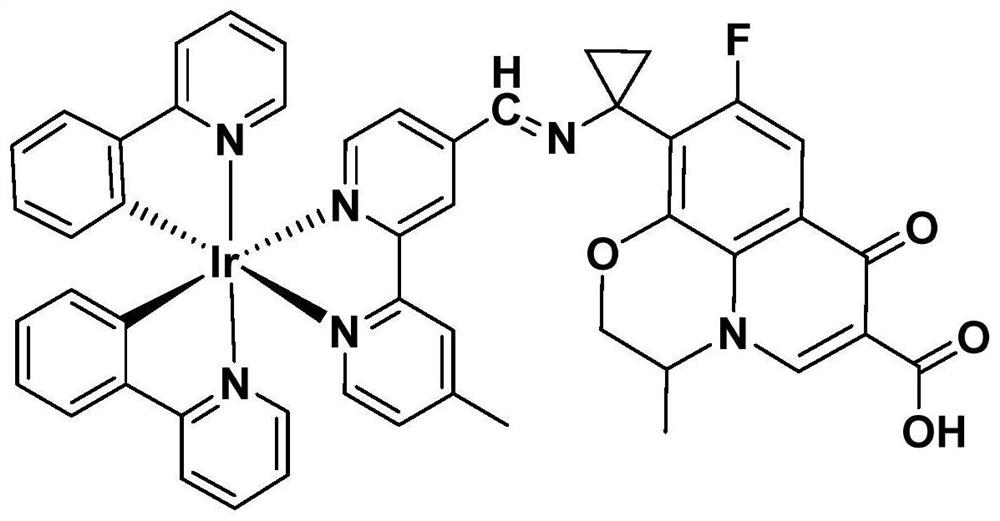
[0060] A preparation method of pazufloxacin iridium complex (Ir-PAZ), comprising the following steps:
[0061] The pazufloxacin iridium complex prepared in embodiment 1 is [Ir(ppy) 2 (mbpyPAZ)]PF 6 .
[0062] Preparation of 4'-methyl-2,2'-bipyridine-4-carbaldehyde:
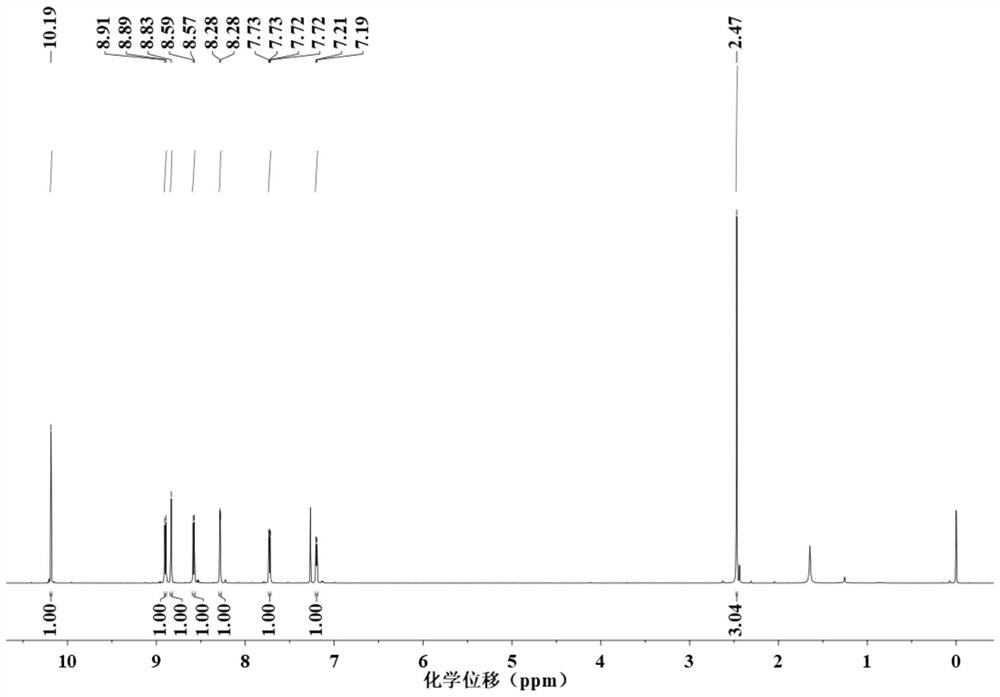
[0063] 2mmol of 4,4'-dimethyl-2,2'-bipyridine (dmb) and 2.5mmol of selenium dioxide (SeO 2 ) was added into the mixture of 20mL dioxane, and then the solution was heated under reflux for 24 hours under argon. After the reaction was completed, the solution was cooled to room temperature, the insoluble matter was removed by suction filtration, and the dioxane was removed by rotary evaporation under reduced pressure. Add 80 mL of ethyl acetate to the solid in the flask, heat at reflux at 60°C for 2 hours, and then remove the insoluble solid by suction filtration while hot. After the filtrate is cooled to room temperature, wash the filtrate three times with 40mL of 0.1M sodium carbonate, then extract the product t...
Embodiment 2
[0078] A preparation method of pazufloxacin iridium complex (Ir-PAZ), comprising the following steps:
[0079] The pazufloxacin iridium complex prepared in embodiment 2 is [Ir(ppy) 2 (mbpyPAZ)]PF 6 .
[0080] Preparation of 4'-methyl-2,2'-bipyridine-4-carbaldehyde:
[0081] 3 mmol of 4,4'-dimethyl-2,2'-bipyridine (dmb) and 3 mmol of selenium dioxide (SeO 2 ) was added into the mixture of 35mL dioxane, and then the solution was heated under reflux for 24 hours under argon gas. After the reaction was completed, the solution was cooled to room temperature, the insoluble matter was removed by suction filtration, and the dioxane was removed by rotary evaporation under reduced pressure. Add 120 mL of ethyl acetate to the solid in the flask, heat at reflux at 60°C for 2 hours, and then remove the insoluble solid by suction filtration while it is hot. After the filtrate is cooled to room temperature, wash the filtrate three times with 40mL of 0.1M sodium carbonate, then extract th...
Embodiment 3
[0089] A preparation method of pazufloxacin iridium complex (Ir-PAZ), comprising the following steps:
[0090] The pazufloxacin iridium complex prepared in embodiment 3 is [Ir(bzq) 2 (mbpyPAZ)]PF 6 .
[0091] Preparation of 4'-methyl-2,2'-bipyridine-4-carbaldehyde:
[0092] 2mmol of 4,4'-dimethyl-2,2'-bipyridine (dmb) and 2.5mmol of selenium dioxide (SeO 2 ) was added into the mixture of 20mL dioxane, and then the solution was heated under reflux for 24 hours under argon. After the reaction was completed, the solution was cooled to room temperature, the insoluble matter was removed by suction filtration, and the dioxane was removed by rotary evaporation under reduced pressure. Add 80 mL of ethyl acetate to the solid in the flask, heat at reflux at 60°C for 2 hours, and then remove the insoluble solid by suction filtration while hot. After the filtrate is cooled to room temperature, wash the filtrate three times with 40mL of 0.1M sodium carbonate, then extract the product t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com