Polymer with electrochromic and thermochromic properties as well as preparation method and application thereof
A thermochromic and electrochromic technology, applied in the field of color-changing materials, can solve the problem of not having both electrochromic and thermochromic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
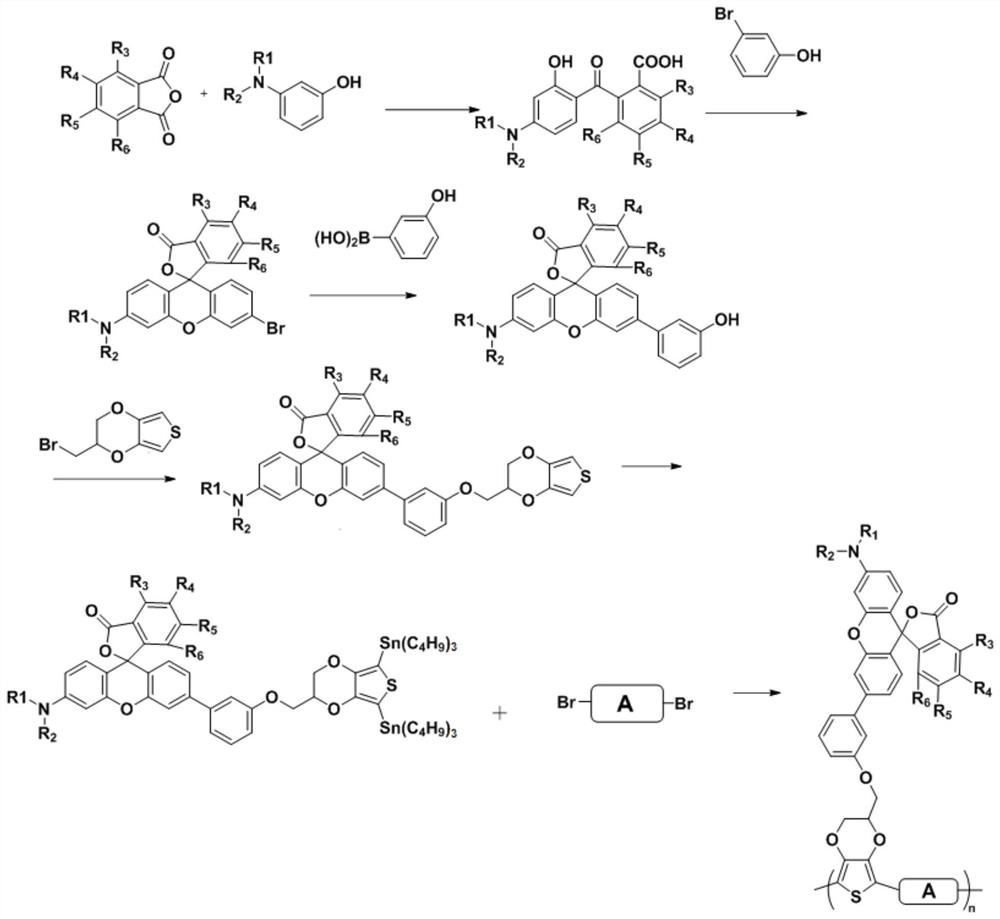
[0053] The embodiment of the present invention also provides a method for preparing a polymer having both electrochromic and thermochromic properties, which includes the steps of:
[0054] S1, the substituted phthalic anhydride and 3-(N-R 1 , N-R 2 Substituted ammonia)-1-phenol is dissolved in the first organic solvent and reacted to obtain 2-(4'-(N-R 1 , N-R 2 Substituted amino)-2'-hydroxybenzoyl) substituted benzoic acid;
[0055] S2, 2-(4'-(N-R 1 , N-R 2 Substituted ammonia)-2'-hydroxybenzoyl) substituted benzoic acid, m-bromophenol, and sulfuric acid are mixed and then reacted to obtain an intermediate compound;
[0056] S3, dissolving the intermediate compound and 3-hydroxyphenylboronic acid in a second organic solvent to undergo a Suzuki coupling reaction to obtain a rhodamine structure derivative containing a hydroxyl group;
[0057] S4. Dissolving rhodamine structural derivatives and 2-bromomethyl-3,4-ethylenedioxythiophene in a third organic solvent, and reactin...
Embodiment 1
[0084] Synthesis of polymer A-1-1 containing rhodamine structural units and phenyl groups substituted with alkoxyalkyl chains on 3,4-ethylenedioxythiophene (EDOT).
[0085] Its synthetic reaction formula is as follows:
[0086]
[0087] The preparation steps of polymer A-1-1 corresponding to the above synthetic reaction formula are as follows:
[0088] ((1-1), (1-2), (1-3) etc. behind the reactant in the following steps correspond to the structural formula in the above-mentioned synthetic reaction formula, and the same applies to Embodiment 2-25)
[0089] (1) Dissolve 74.0g (0.5mol) of phthalic anhydride (1-1) and 68g (0.5mol) of 3-dimethylaminophenol (1-2) in 1000mL of toluene, and react at 110°C under nitrogen After 4 hours, 180 mL of 10 mol / L sodium hydroxide solution was added dropwise, and heated at 100°C for 7 hours. After the reaction, the reaction mixture was poured into ice water, neutralized with hydrochloric acid, extracted with chloroform, the organic phases w...
Embodiment 2
[0096] Synthesis of Polymer A-2-1 Containing Rhodamine Structural Units and Naphthyl in Branched Chains of 3,4-Ethylenedioxythiophene (EDOT)
[0097] The synthetic reaction formula of compound (1-10) is with embodiment 1, and the different reaction formula of embodiment 1 is as follows:
[0098]
[0099] The preparation steps of polymer A-2-1 are as follows:
[0100] The preparation steps of compound (1-10) are the same as those in Example 1, and will not be repeated here.
[0101] 11.7g (10mmol) of compound (1-10) and 2.8g (10mmol) of 2,6-dibromonaphthalene (2-1) were dissolved in 100mL of dry toluene, degassed with nitrogen for 20 minutes, and added 0.22g (0.2 mmol) tetrakistriphenylphosphinepalladium, stirred at 110°C for 24h, cooled to room temperature, poured into a large amount of methanol, and filtered. The filter cake was extracted with methanol, acetone, normal hexane, and chloroform Soxhlet, and the chloroform layer was concentrated to obtain 6.3 g of 3,4-ethyle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com