Application of CD146 + mesenchymal stem cell subpopulation in preparation of medicine for preventing and treating premature ovarian failure
A technology of premature ovarian failure and mesenchymal stem cells, which is applied in the application field of medicine, can solve the problems such as the lack of therapeutic effect of CD146+ mesenchymal cell subsets on premature ovarian failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
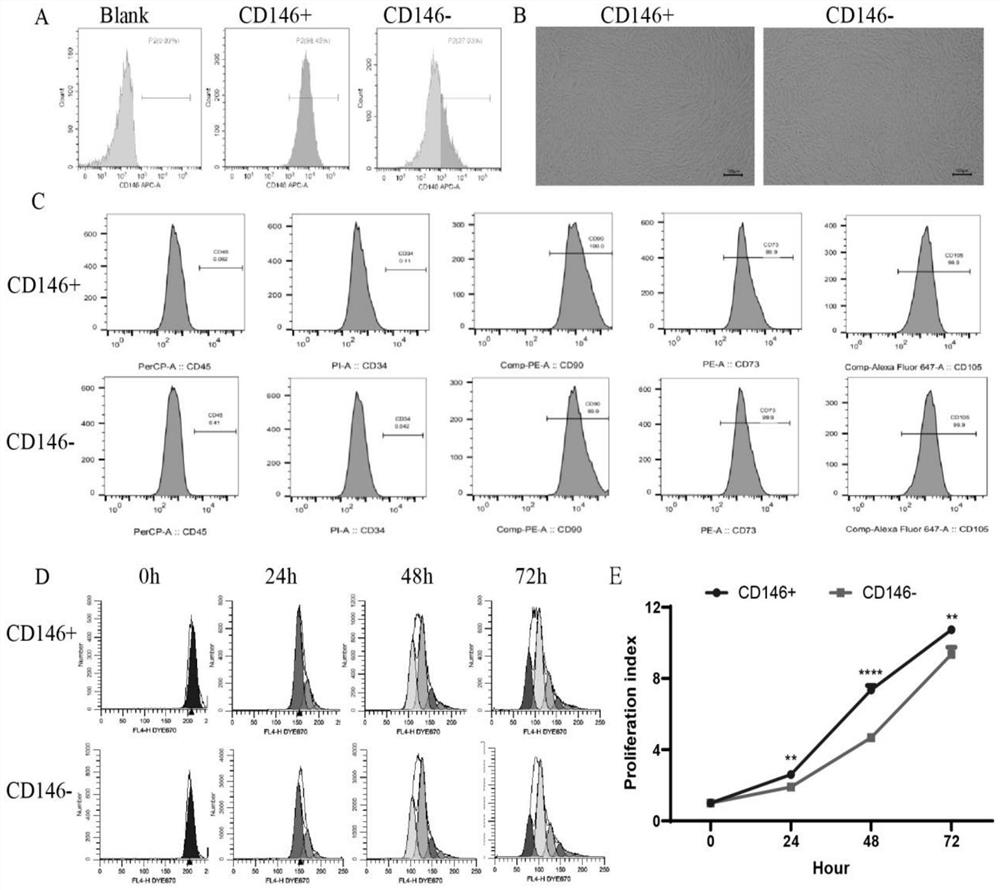
[0031] Preparation and isolation method of CD146+ / - mesenchymal stem cell subpopulation
[0032] 1. Materials and methods:
[0033] 1) Cell culture
[0034] The first passage of human umbilical cord-derived mesenchymal stem cells (UC-MSCs) was provided by the Shandong Provincial Cord Blood Bank (Qingdao, China). Cells were cultured with 90% minimal essential medium (αMEM, BI) and 10% fetal bovine serum (Gibco, Australia) at 37°C, 5% CO 2 After further passage (P), MSCs were harvested with trypsin-edta (Gibco, Australia), counted, and re-seeded into 100 mm cell culture dishes at a density of 20,000 cells / cm2.
[0035] 2) Isolation of CD146+MSCs and CD146-MSCs
[0036] Umbilical cord MSCs were separated into CD146+ / - subsets by MACS (Magnetic Activated Cell Sorting). Cells were incubated with FcR blocking reagent and CD146 microbeads (CD146 Bead Kit; Miltenyi Biotec) before magnetic separation. The LS column was used to sort CD146+ cells, and the CD146- cells in the LS colu...
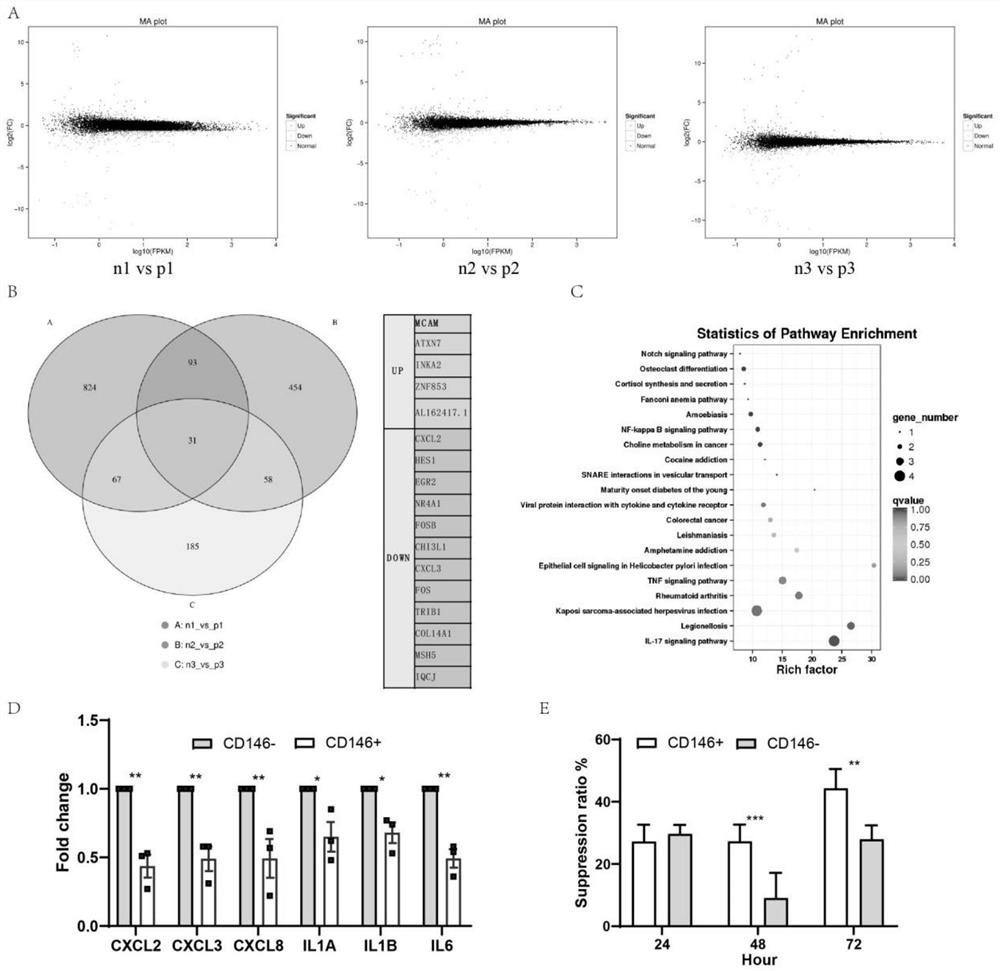
Embodiment 2
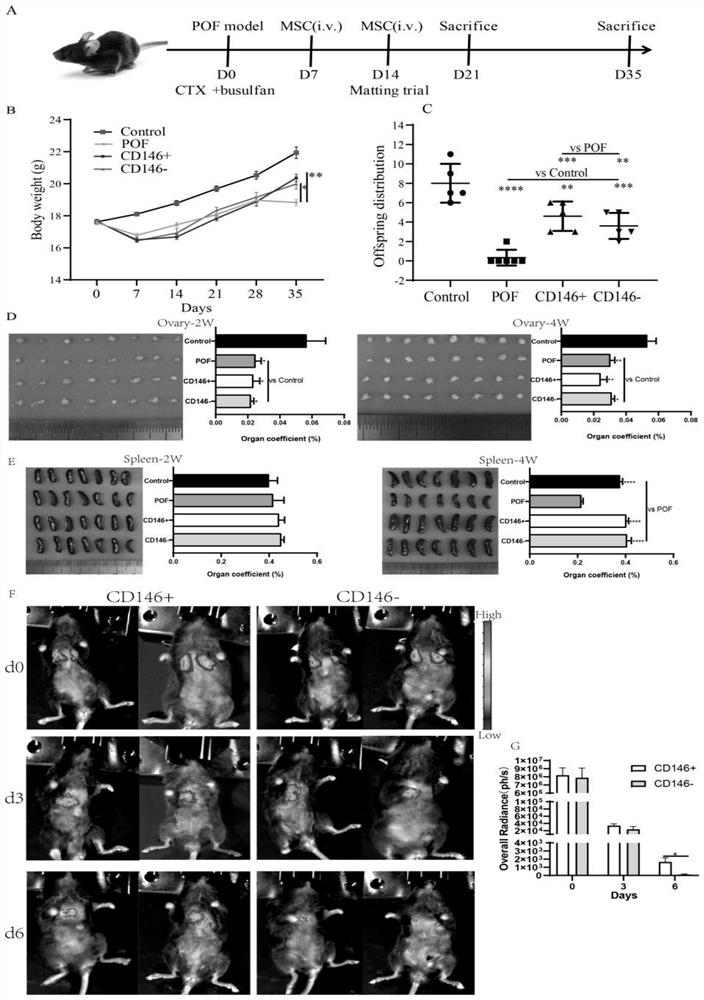
[0047] Construction of premature ovarian failure (POF) model in mice and cell therapy
[0048] 1. In order to establish a premature ovarian failure model, female C57BL / 6 mice aged 6-8 weeks in the experimental group were subcutaneously injected with busulphan (Bu, 30 mg / kg, dissolved in DMSO), and intraperitoneally injected with cyclophosphamide (Cy, 120 mg / kg, dissolved in physiological saline), and the mice in the control group (n=20) were injected with the same amount of DMSO and saline. HE staining results showed that follicle development stopped at the primary follicle stage, resulting in a decrease in antral follicles in POF ovaries. Consistent with the sinus follicle damage, the serum AMH level in the POF group also increased significantly, indicating that the mouse model of premature ovarian failure was successfully established.
[0049] In the POF group (n=20), mice received no cell therapy after chemotherapy. In the CD146+ / - MSCs treatment group (n=20), 1 week afte...
Embodiment 4
[0066] 1. Fertility experiment of premature ovarian failure mouse model after cell therapy
[0067] According to the method described in Example 3, the mouse model of premature ovarian failure was constructed, and two kinds of cell therapy were performed. After the second injection of CD146+ / - MSCs, adult males and females who were confirmed to have fertility were raised in a ratio of 1:2. . The litter size was recorded for each gestation.
[0068] 2. Histological Analysis of Ovaries and Spleen
[0069] At 4w, the ovaries were collected and fixed with 4% formaldehyde. The ovaries were cut into 5 μm sections, stained by ultrasound, and evaluated by light microscope for histology. Count and record as preantral follicles (primitive, primary), preantral follicles (secondary, mature), or atretic follicles. Spleens were processed the same as ovaries for H&E staining. Images were taken under a light microscope to record the histological changes of the spleen.
[0070] 3. Biolum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com