Cefalonium breast injectant as well as preparation method and application thereof
A technology of breast injection and cefuroxime, which is applied in the field of cefuroxime breast injection and its preparation, can solve the problems that the drug concentration cannot be maintained until the perinatal period and the long milk abandonment period, and achieves the problem of milk abandonment period, The effect of short milk abandonment period and high drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The present invention also provides the preparation method of described ceftaroning breast injection, comprises the steps:
[0041] Mix the emulsifier and the oily solvent, and homogenize the obtained mixture for the first time to prepare a homogeneous liquid;
[0042]Mix the homogenized liquid and cefuroxime, and homogenize the obtained mixture for the second time to prepare the cefuroxime breast injection.
[0043] In one specific example, the conditions for the first homogenization include: the pressure is 450bar-550bar. Specifically, the pressure of the first homogenization includes but is not limited to: 450 bar, 460 bar, 470 bar, 480 bar, 490 bar, 500 bar, 510 bar, 520 bar, 530 bar, 540 bar, 550 bar.
[0044] In one specific example, the number of times of the first homogenization is 1.
[0045] In one specific example, the conditions for the second homogenization include: a pressure of 950 bar to 1050 bar. Specifically, the pressure of the second homogenizatio...
Embodiment 1
[0050] Present embodiment is a kind of ceftaroning breast injection, and the composition of every 3g is composed of:
[0051]
[0052] The preparation method of above-mentioned ceftaroning breast injection is as follows:
[0053] (1) Add Span 80, lecithin, and Tween 20 to medium-chain fatty acid glycerides, and homogenize once with a high-pressure homogenizer at a pressure of 500 bar to obtain Liquid A;
[0054] (2) Put the main drug cefuroxime into the liquid A, homogenize twice at low temperature with a high-pressure homogenizer, and the pressure is 1000 bar to obtain the cefuroxime breast injection.
Embodiment 2
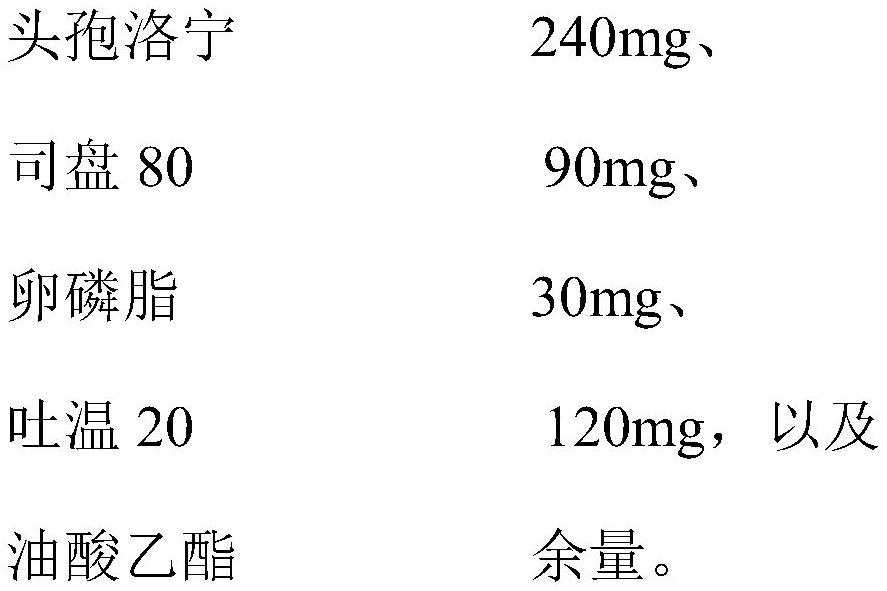
[0056] Present embodiment is a kind of ceftaroning breast injection, and the composition of every 3g is composed of:
[0057]
[0058] The preparation method of above-mentioned ceftaroning breast injection is as follows:
[0059] (1) Add Span 80, lecithin, and Tween 20 into ethyl oleate, homogenize once with a high-pressure homogenizer at a pressure of 500 bar, and obtain Liquid A;
[0060] (2) Put the main drug cefuroxime into the liquid A, homogenize twice at low temperature with a high-pressure homogenizer, and the pressure is 1000 bar to obtain the cefuroxime breast injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com