Early diagnosis kit based on combined detection of four items of liver cancer and application
A combined detection and kit technology, applied in the field of immunodetection, achieves the effects of good stability, reduced detection time and cost, and easy mixing and combination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1. Preparation of four combined assay kits for liver cancer.
[0036] 1. The preparation method of biotin-labeled antibody is as follows:
[0037] Take biotin-labeled rabbit anti-AKR1B10 polyclonal antibody as an example:
[0038] (1) Replace the rabbit anti-AKR1B10 polyclonal antibody to be labeled with a desalting column into a 1x PBS buffer.
[0039] (2) Mix the antibody in step (1) with biotin at a concentration of 10mM, use a molecular ratio of 20:1 between biotin and antibody, and react with gentle shaking at room temperature (18-25°C) for 2 hours.
[0040] (3) Replace the labeled antibody solution with a desalting column into a 1x PBS solution to remove free biotin and store at -80°C to prepare the desired biotin-labeled antibody.
[0041] The preparation of other biotin-labeled antibodies can be carried out by referring to the above method.
[0042] 2. The preparation method of acridinium ester-labeled antibody is as follows:
[0043] Take acridinium...
Embodiment 2
[0059] Example 2. Detection steps of the four combined assay kits for liver cancer.
[0060] 1. The detection steps of the first reagent, the second reagent and the fourth reagent are as follows:
[0061] (1) Add an appropriate volume of each sample to three reaction cups, and add corresponding volumes of AKR1B10, AFP and DCP calibrator and quality control products to the reaction cups.
[0062] (2) Add the biotin antibody and acridinium ester antibody of AKR1B10, AFP and DCP into the corresponding cuvettes respectively, and the addition amount is 100uL respectively.
[0063] (3) Incubate the cuvettes at 37°C for 10 minutes, then add 30uL streptavidin magnetic beads to each cuvette, and incubate at 37°C for 20 minutes.
[0064] (4) Adsorb the magnetic beads under the action of magnetic force, absorb the liquid in the cuvette, and wash repeatedly 3 times.
[0065] (5) Under the action of the pre-excitation solution and the excitation solution, the acridinium ester is excited ...
Embodiment 3
[0076] Example 3. The normal reference value of the four joint assay kits for liver cancer.
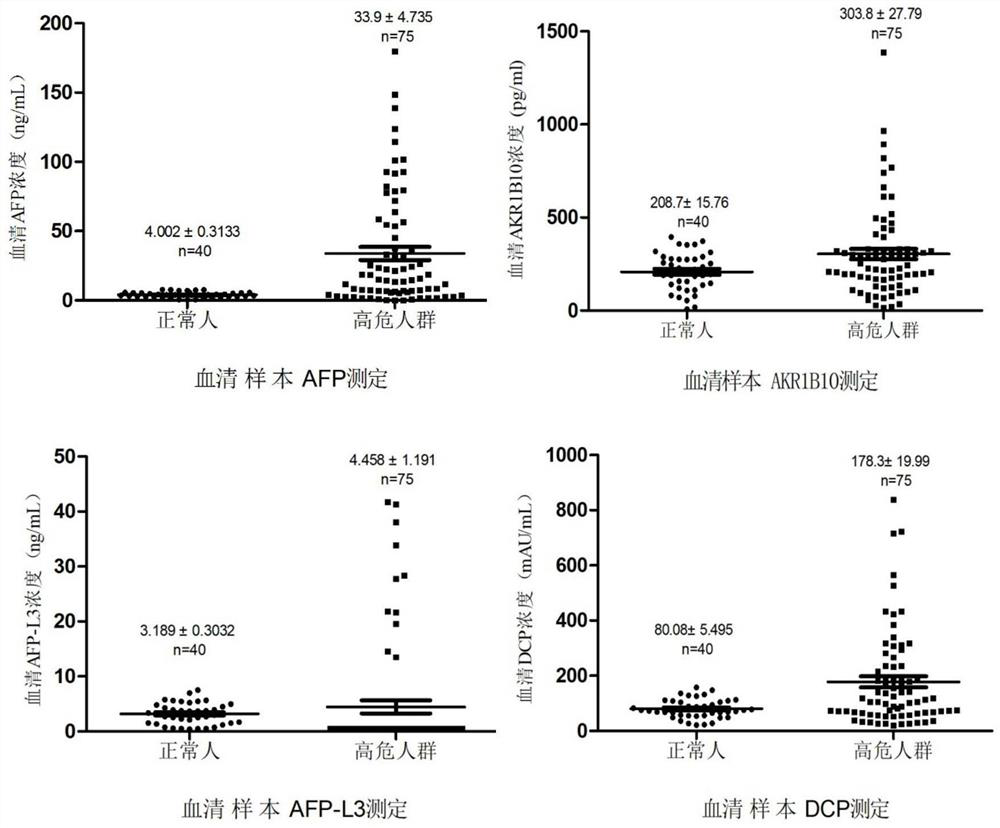
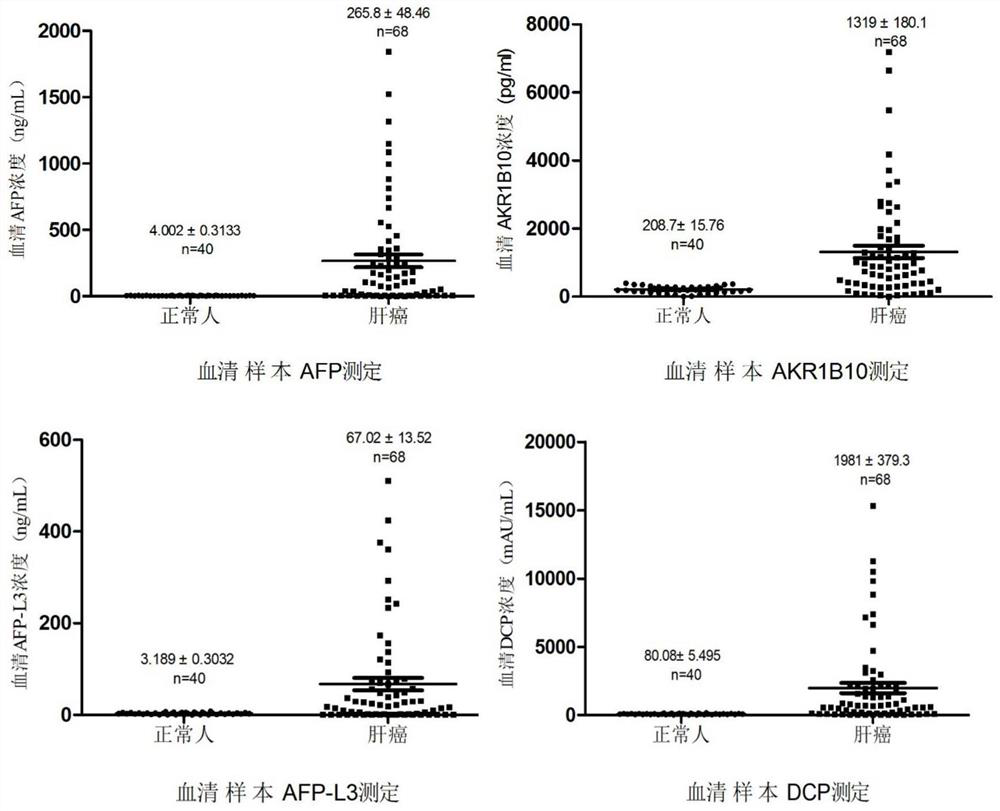
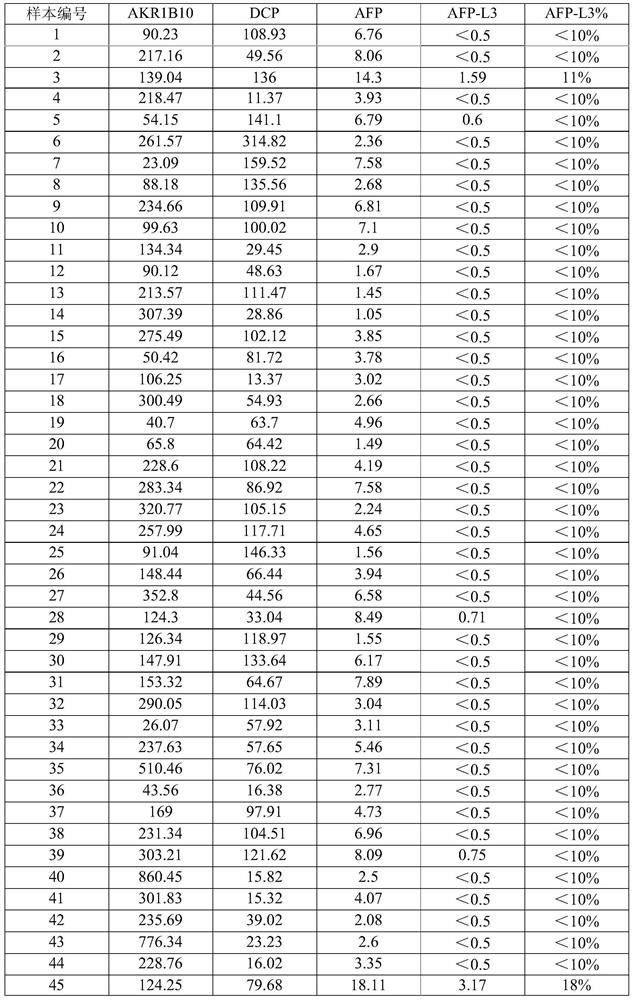
[0077] Adopt the detection method identical with embodiment 2, select 228 routine healthy normal person samples, determine normal reference value according to 95% percentile (P 95 =(228+1)×95%=217). According to the order of sample concentration, in 228 serum samples, the concentration of AKR1B10 corresponding to P95=217 is 373.5pg / mL, the concentration of DCP is ≤161.45mAU / mL, the concentration of AFP is 8.7ng / mL, and the ratio of AFP-L3% ≤10%, see Table 1 for detailed results, (AFP-L3% is the concentration ratio of AFP-L3 and AFP).
[0078] Table 1. Measured values of serum samples of 228 healthy normal persons
[0079]
[0080]
[0081]
[0082]
[0083]
[0084] Therefore, it was determined that the normal reference value of AKR1B10 was ≤350 pg / mL, the normal reference value of AFP was ≤8.7 ng / mL, the normal reference value of AFP-L3 / AFP was ≤10%, and the normal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com