New application of compound of angiotensin II receptor antagonist metabolite and NEP inhibitor
A receptor antagonist, angiotensin technology, applied in the field of drug application, can solve problems such as untargeted drugs, and achieve the effect of good antihypertensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Preparation of AHU377 free acid:
[0092] Add 2.1g of AHU377 calcium salt and 40mL of isopropyl acetate into a 250mL single-necked bottle, add 4.5mL of 2mol / L hydrochloric acid at room temperature and stir to dissolve. Separate the liquid, collect the organic layer, and wash the organic layer twice with 20 mL of water; desolvate under reduced pressure at 35°C to obtain the free acid of AHU377.
Embodiment 2
[0094] Preparation of complex:
[0095]
[0096] At room temperature, add 2.36g of AHU377 free acid, 2g of EXP3174 and 40mL of acetone obtained according to the method in Example 1 into a 250mL three-neck flask, and dissolve; add 1.3 equivalents of calcium hydroxide solid and 1mL of water relative to AHU377 at room temperature, and stir at room temperature 10h, add 40mL of acetone, react for another 8h, filter through a Buchner funnel under nitrogen protection, rinse the solid with acetone to obtain a white solid, dry in vacuum at 35°C for 8h, and dry to obtain 3.5g of solid, the purity of which is 99% by HPLC. %, the molar ratio of EXP3174 and AHU377 in the obtained product is 1:1 through content test calculation.
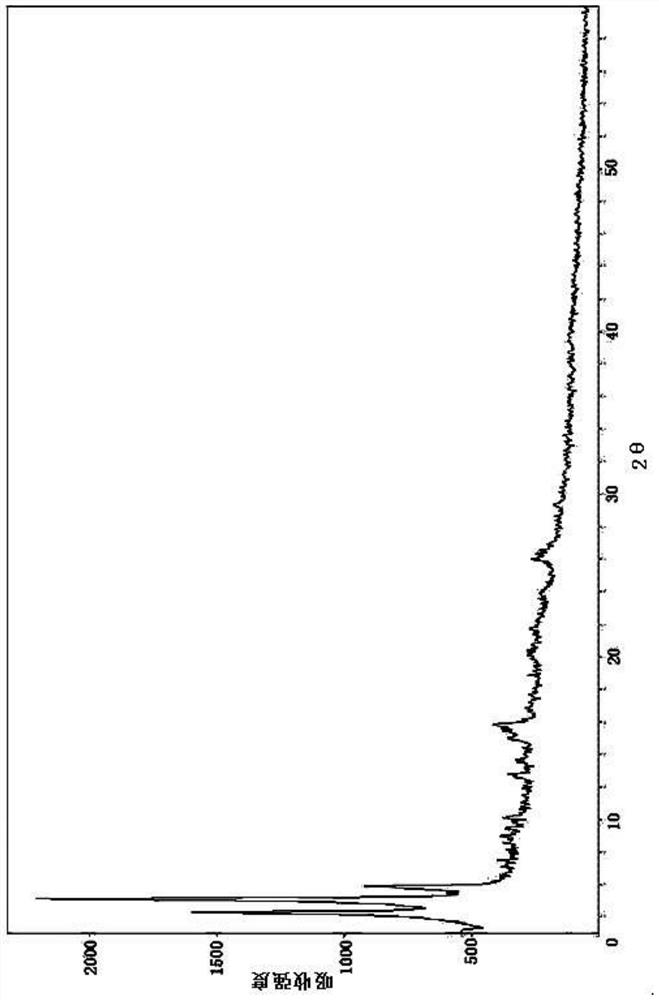
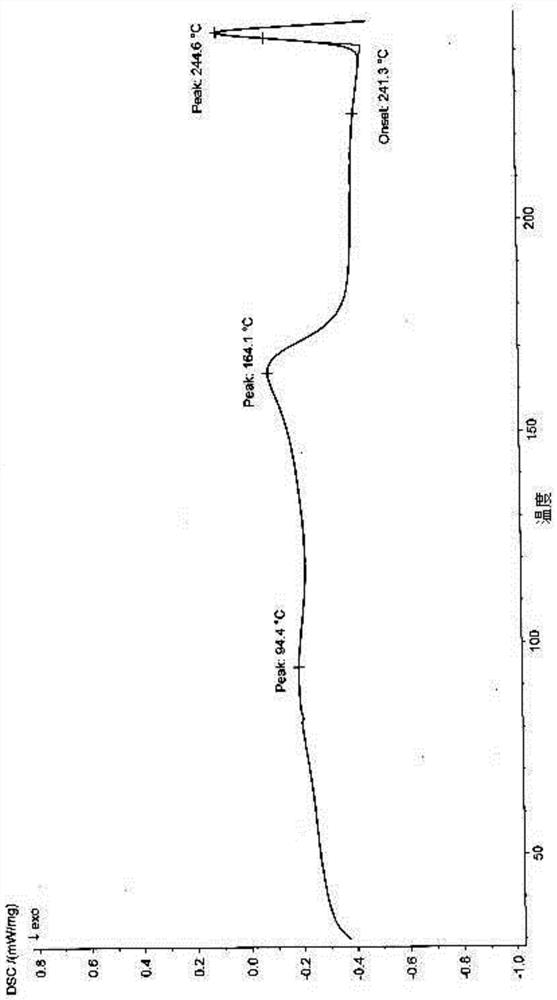
[0097] The X-ray powder diffraction spectrogram of gained product is as figure 1 As shown, the DSC spectrum is as figure 2 shown.
[0098] By comparing with the XRD patterns of EXP3174 and AHU377 calcium, it is found that there are obvious differences in t...
Embodiment 3
[0111] Preparation of complex:
[0112]
[0113] At room temperature, add 2.36g of AHU377 free acid, 2g of EXP3174 and 40mL of acetone obtained according to the method in Example 1 into a 250mL three-necked flask, and dissolve it; Stir at ℃ for 6 hours, add 40 mL of acetone, react for another 8 hours, filter through a Buchner funnel under nitrogen protection, rinse the solid with acetone to obtain a white solid, dry in vacuum at 50 °C for 8 hours, and dry to obtain 3.1 g of solid, which passed the content test The calculation shows that the molar ratio of EXP3174 and AHU377 in the obtained product is 1:1.
[0114] The DSC spectrogram of gained product is as Figure 5 shown.
[0115] Elemental analysis, found value: C: 58.51%; H: 5.41%; N: 10.25%. Theoretical value (according to (EXP3174·AHU377) 3-·1.5Ca2+·2H2O): C: 58.68%; H: 5.46%; N: 10.41%.
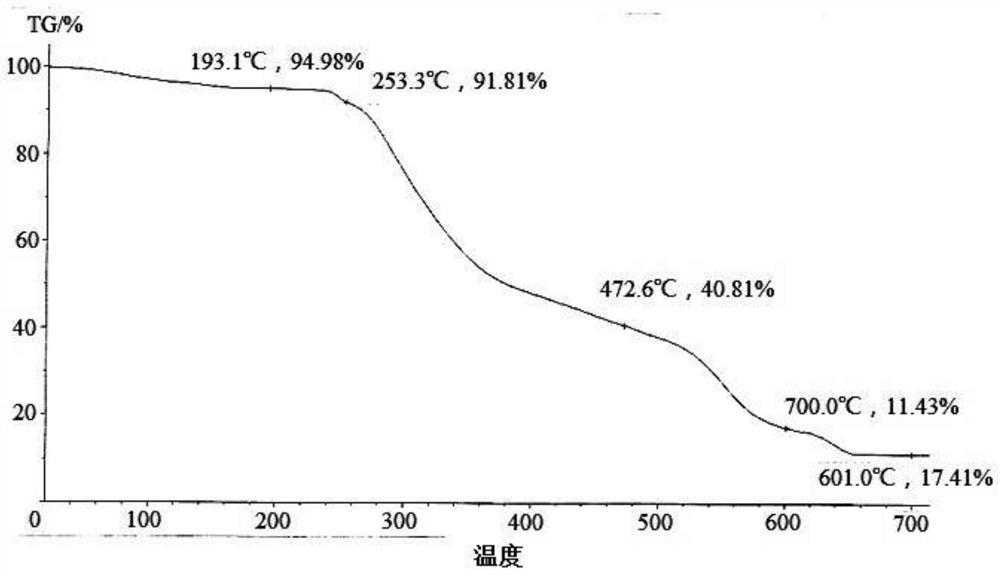
[0116] The TG spectrogram of gained product is as Image 6 As shown, the water content of the obtained product measured by th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com