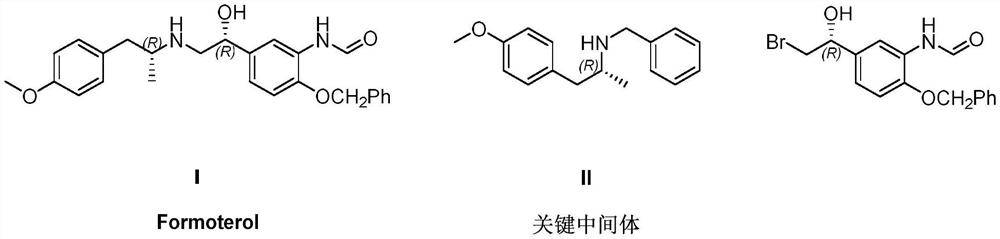
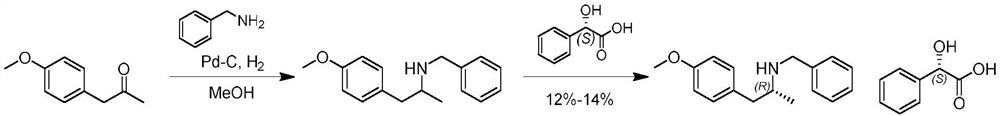
Preparation method of (R)-N-benzyl-1-(4-methoxyphenyl) propan-2-amine
A technology of methoxyphenyl and benzyl, which is applied in the field of preparation of -N-benzyl-1-propan-2-amine, can solve the problems of low resolution yield and high production cost, and achieve the goal of reducing dangerous reagents Use, suitable for large-scale production, avoiding the effect of low yield of chiral resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
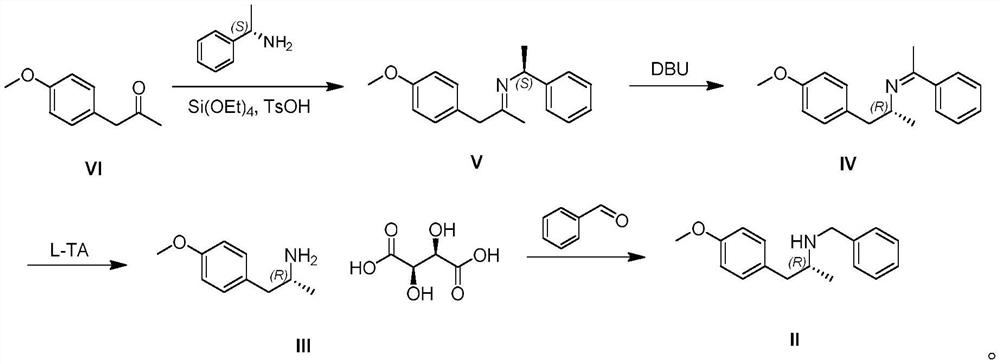
[0021] A synthetic route of (R)-N-benzyl-1-(4-methoxyphenyl)propan-2-amine disclosed by the present invention is as follows:
[0022]
[0023] Specific steps are as follows:
[0024] S1, the structural formula is Compound VI and S-methylbenzylamine carry out condensation reaction to obtain structural formula: Compound V, tetraethylorthosilicate and p-toluenesulfonic acid were added during the reaction.
[0025] The preparation steps of compound V are as follows: add S-methylbenzylamine (12.1g, 0.10mol), tetraethylorthosilicate (10.4g, 0.05mol), p-toluenesulfonic acid (0.76g, 0.004 mol) and 2-methyltetrahydrofuran (100mL), cooled with ice water until the internal temperature was less than 30°C, added p-methoxyphenylacetone (20.0g, 0.12mol) dropwise, heated to 90°C after the dropwise addition, and reacted 17 Hours, TLC (thin layer chromatography) control reaction is completed, cooled to 30 ° C, pad Celite filter, filter cake with 200mL of methyl tert-butyl ether, combine...
Embodiment 2
[0033] The difference between the preparation method disclosed in this example and Example 1 is that S-methylbenzylamine, tetraethylorthosilicate, p-toluenesulfonic acid and tetrahydrofuran are sequentially added in step S1.
Embodiment 3
[0035] The difference between the preparation method disclosed in this example and Example 1 is that in step S4, the free compound III is dissolved in methanol, and then benzaldehyde and palladium carbon are added.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com