Amidinourea lead compound and synthesis method thereof
A lead compound and amidinourea technology, applied in the field of amidinourea lead compounds and their synthesis, can solve the problems of denaturation, DNA instability and the like, and achieve the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
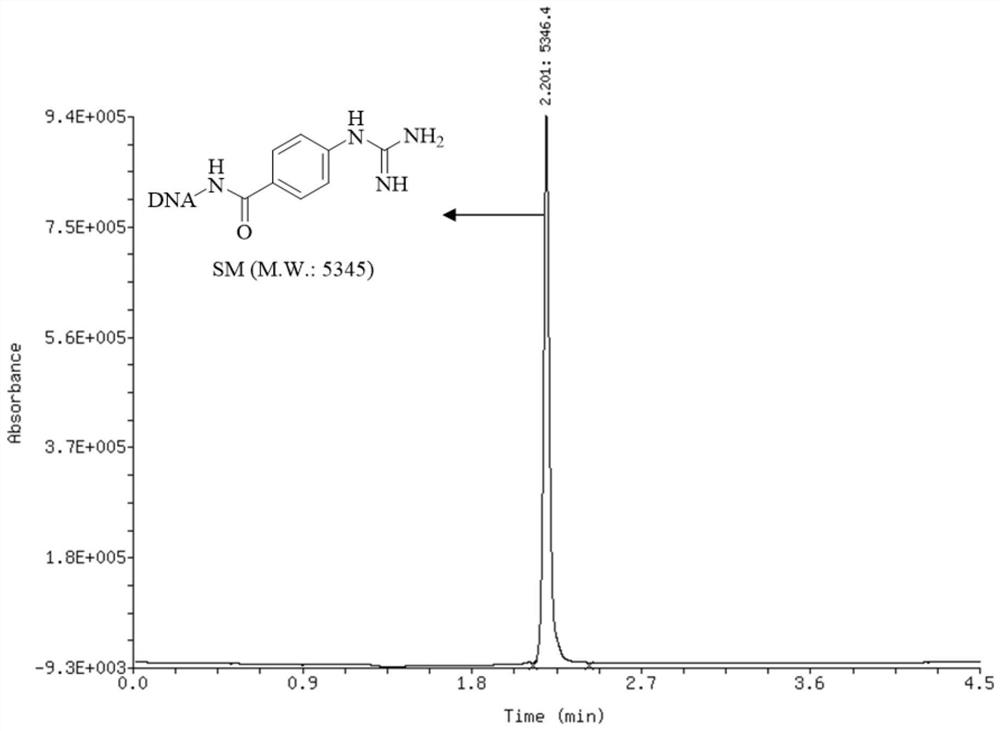
[0117] Embodiment 1, the synthesis of oligomeric nucleic acid-guanidine compound raw material
[0118]
[0119] The oligonucleotide used in the present invention-NH 2 (DNA-NH 2 ) is composed of oligonucleotide (structure as follows, supplier GenScript, molecular weight 4937) with DMT-MM-BF 4 (supplier: Suzhou Capwell Pharmaceutical Technology Co., Ltd.) obtained by condensation of condensing agent and Fmoc-NH-PEG4-propionic acid (supplier: Bi De) by conventional methods (Li Y., Gabriele E., Samain F., Favalli N., Sladojevich F., Scheuermann., Optimized Reaction Conditions for Amide Bond Formation in DNA Encoded Combinatorial Libraries, Neri D. ACS Comb. Sci. 2016, 18, 8, 438–443.).
[0120]
[0121] Oligonucleotide (molecular weight 4937)
[0122] The synthetic method of oligomeric nucleic acid-guanidine compound raw material of the present invention comprises the steps:
[0123] 100nmol oligo-NH 2Dissolve it in deionized water to prepare an aqueous solution with a ...
Embodiment 2
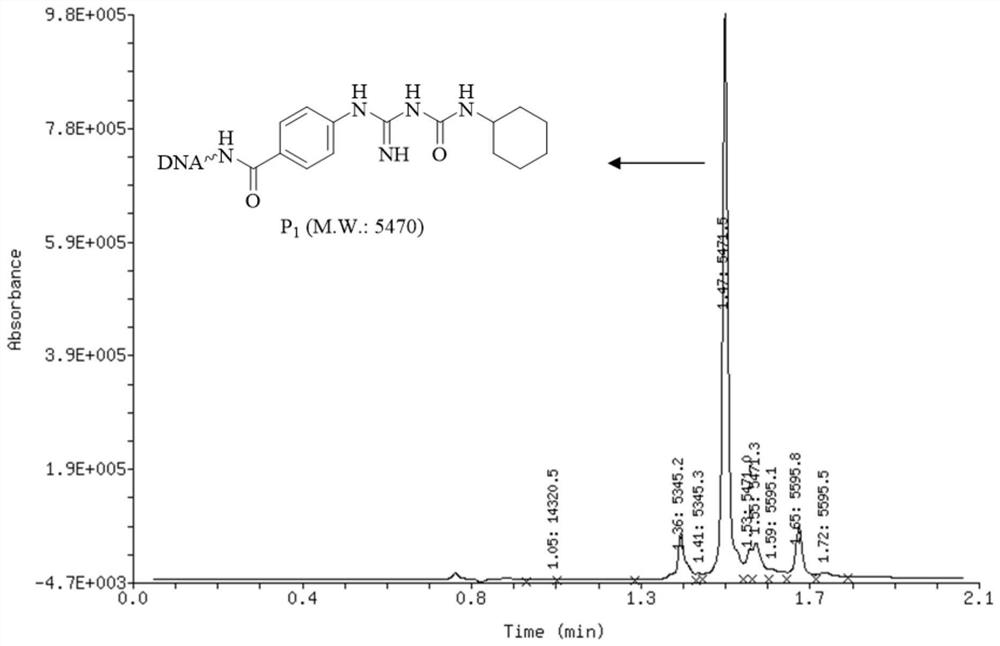
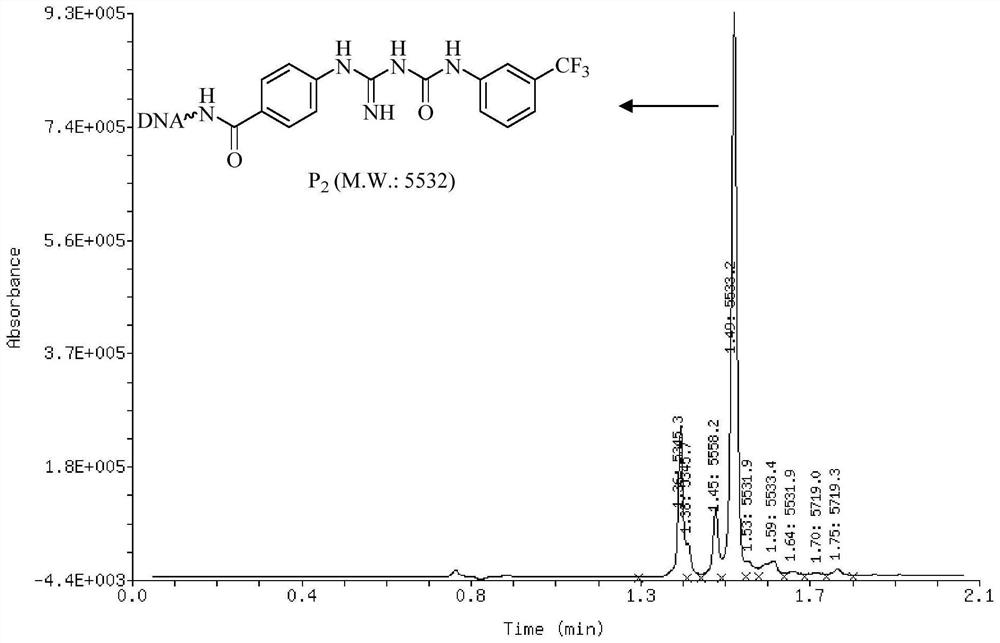
[0126] Embodiment 2, the general synthetic method of oligomeric nucleic acid-guanidinourea compound
[0127] The synthetic reaction formula of universal oligomeric nucleic acid-guaninourea compound is as follows:
[0128]
[0129] In 0.5nmol oligonucleotide-guanidine compound aqueous solution (aqueous solution with a concentration of 1mmol / L, 1 equivalent, 0.5nmol, 0.5μL), add a sodium tetraborate buffer solution (1.0μL, pH=12.5) with a concentration of 250mmol / L and Isocyanate acetonitrile solution (isocyanate concentration is 1mol / L, 1000 equivalents, 500nmol, 0.5μL), react at 90°C, and add 500nmol isocyanate acetonitrile solution every 3 hours during the reaction (isocyanate concentration is 1mol / L, 1000 equivalents , 500nmol, 0.5μL), supplemented twice, a total of 1500nmol of isocyanate, a total of 22 hours of reaction. After the reaction was completed, a 10% total volume of 5 mol / L sodium chloride aqueous solution was added to the reaction liquid. Then, continue to a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com