Synthetic method and application of o-phenol sulfimine compound of on-dna
A technology of phenol sulfimide and synthesis method, which is applied in the field of biomedicine and can solve the problems of limited diversity of molecular chemical structures and limited reaction types, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] A method for synthesizing an on-DNA o-phenol sulfimine compound, comprising:
[0059] S01, providing a substrate and a thio reagent, the structural formula of the substrate is DNA-Ar-O-NH-R 1 , the thio reagent is an N-alkylthio substituted isoindole-1,3-dione compound; wherein, DNA is a nucleotide chain, Ar is selected from a benzene ring, an aromatic ring or a heterocycle, and R 1 One selected from alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, alkoxy, aryl, heteroaryl;
[0060] S02, reacting the substrate and the thio reagent in a solvent system containing an inorganic buffer to obtain on-DNA ortho-phenol sulfimine compounds.
[0061] In step S01, the structural formula of the substrate is DNA-Ar-O-NH-R 1 , wherein, DNA is a single-stranded or double-stranded nucleotide chain obtained by polymerizing artificially modified and / or unmodified nucleotide monomers, and the DNA can pass through carboxyl groups, amine groups, C-C bonds or C-N bonds, etc. A variety of ...
Embodiment 1
[0091] 1. Synthesis of On-DNA N-phenoxyamide compound (3)
[0092] The synthetic route is as follows:
[0093]
[0094] Concrete synthetic steps are as follows:
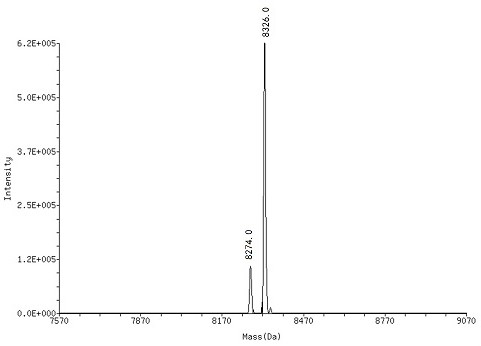
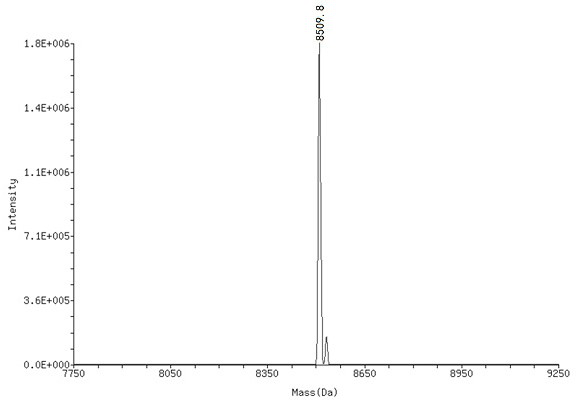
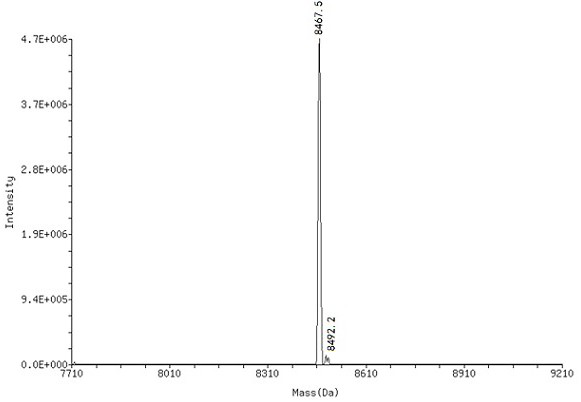
[0095] Compound (1) was dissolved in borate buffer (0.1 M, pH = 9.4) to prepare a solution of compound (1) with a final concentration of 1.0 mM, using DMTMM as a condensing agent, and carboxylic acid derivatives (2 ) reaction to obtain the corresponding On-DNA compound (3), after the completion of the reaction, use ethanol precipitation treatment (specifically: add 5 M sodium chloride solution with 10% of the total reaction volume, 2.5 times the volume of -20 ° C storage without Water ethanol, stand at -20°C for 1 h, centrifuge at 13,300 rpm for 15 min at 4°C), after HPLC purification and MS detection, the target product - On-DNA compound (3), freeze-dried and directly used in the next step reaction.
[0096] 2. Synthesis of On-DNA o-phenol sulfimine compound (6) or (7)
[0097] The synthetic route is as follo...
Embodiment 2
[0108] In this example, the compound (6b) synthesized in Example 1 was taken as a representative, and the effects of reaction temperature, reaction time, organic solvent, and inorganic buffer on the reaction yield were explored according to the method steps of Example 1.
[0109] The synthetic route is as follows:
[0110]
[0111] Table 2 shows the test results. As shown in the results, the reaction temperature, reaction time, organic solvent, and inorganic buffer all have different degrees of influence on the reaction yield.
[0112] Wherein, the reaction temperature of embodiment 1,6,7 is room temperature, and the reaction time is 16h, all adopts PBS damping fluid, and its difference is only to have adopted different organic solvent, and the organic solvent of embodiment 1 is DMSO, and its product Rate is 71%, greater than embodiment 6 and 7, shows to adopt the solvent system that is made up of PBS damping fluid and DMSO, and its productive rate can guarantee at higher l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com