IDO inhibitor, preparation method, pharmaceutical composition and application
A technology of inhibitors and compositions, applied in the direction of drug combinations, antipyretics, anti-inflammatory agents, etc., can solve the problems of low selectivity and weak IDO2 inhibitory activity, and achieve a simple preparation method, wide application, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
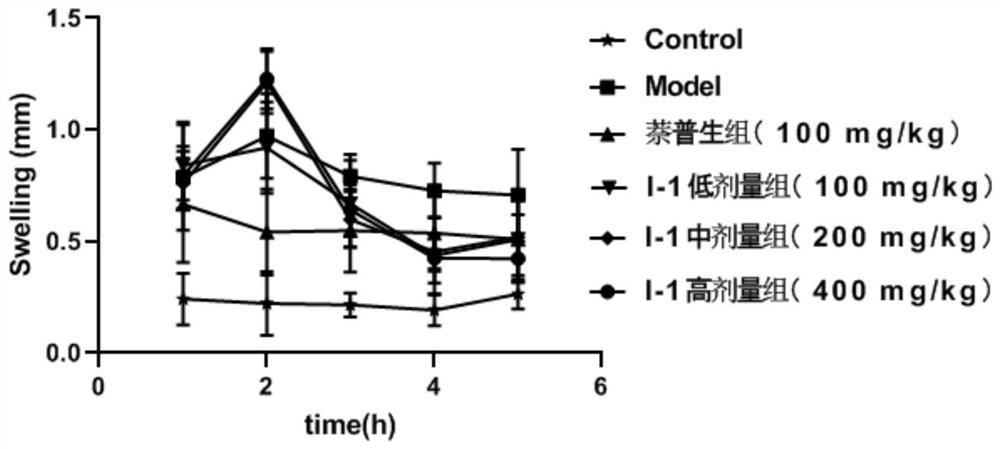
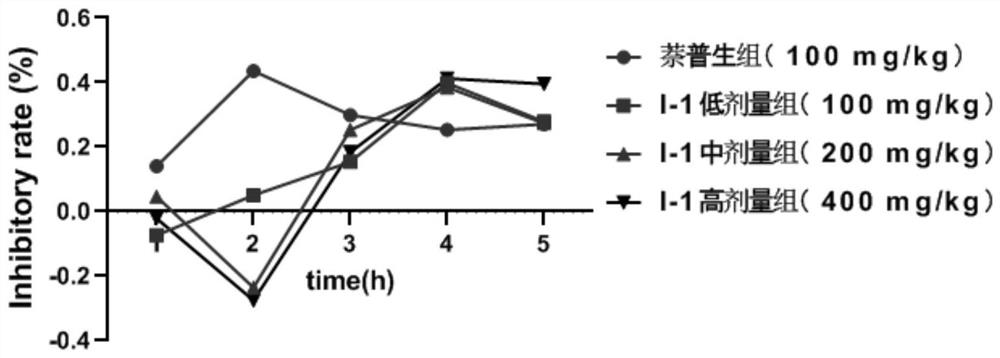
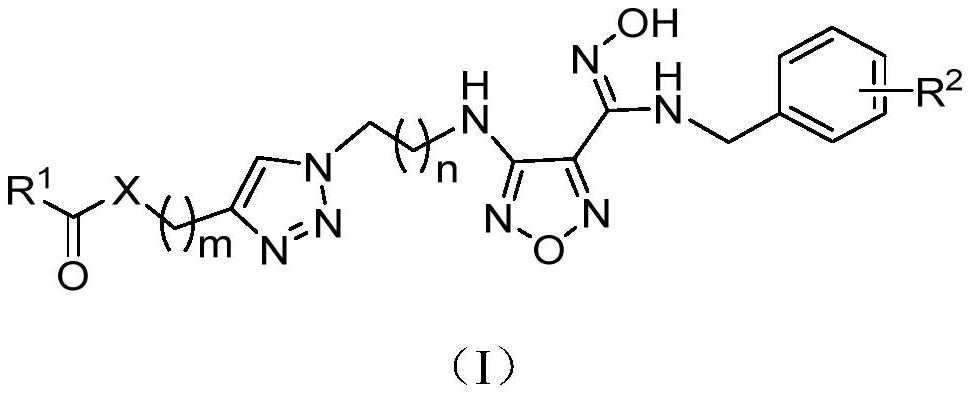
Image
Examples
Embodiment 1
[0050] Example 1: (Z)-N-((1-(2-((4-(N-(3-bromobenzyl)-N'-hydroxyformamidine)-1,2,5-oxadiazole- Synthesis of 3-yl)amino)ethyl)-1H-1,2,3-triazol-4-yl)methyl)picolinamide (I-1)
[0051] Synthesis of (Z)-4-amino-N-(3-bromobenzyl)-N'-hydroxy-1,2,5-oxadiazole-3-carboxamidine (IV-1)
[0052] Into the 2L three-necked flask was sequentially added raw material (Z)-4-amino-N'-hydroxy-1,2,5-oxadiazole-3-carbimidoyl chloride (II-1, 21.0g, 129.7mmol), 3-Bromobenzylamine (III-1, 20.0 g, 108.1 mmol) and 95% ethanol (500 mL) were stirred, and 200 mL of Na was added to the reaction solution. 2 CO 3 The solution (17.2 g, 162.0 mmol) was heated to 60° C. with heating and stirring for 5 hours, and the TLC monitoring (petroleum ether: ethyl acetate=5: 1) was completed; the reaction solution was transferred to a 2L eggplant-shaped bottle, and evaporated under reduced pressure. Ethanol, then add 500 mL of water, stir at 25 ° C for 1 hour, suction filter, wash the filter cake with water (100 × 3 mL),...
Embodiment 2
[0065] Example 2: (Z)-N-((1-(2-((4-(N-(3-bromobenzyl)-N'-hydroxyformamidine)-1,2,5-oxadiazole- Synthesis of 3-yl)amino)ethyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-pyrrole-2-carboxamide (I-2)
[0066] With compound X-1 (0.10 g, 0.26 mmol), N-(propyl-2-yn-1-yl)-1H-pyrrole-2-carboxamide (XI-2, 45 mg, 0.31 mmol) and copper sulfate (8 mg, 0.05 mmol) / sodium ascorbate (20 mg, 0.10 mmol) as raw materials, the same operation as I-1, 60 mg of white solid I-2 was obtained, the yield was 43.8%, m.p. 156.0-158.0 ℃. 1 H-NMR (300MHz, DMSO-d 6), δ(ppm): 11.46(s, 1H), 10.85(s, 1H), 8.52(s, 1H), 7.96(s, 1H), 7.49–7.38(m, 2H), 7.28(t, J= 7.6Hz, 1H), 7.21(d, J=7.8Hz, 1H), 7.08(t, J=7.1Hz, 1H), 6.88–6.81(m, 2H), 6.43(s, 1H), 6.10(s, 1H), 4.66–4.53 (m, 4H), 4.48 (d, J=5.7Hz, 2H), 3.68 (d, J=6.1Hz, 2H).
Embodiment 3
[0067] Example 3: (Z)-N-((1-(2-((4-(N-(3-chlorobenzyl)-N'-hydroxyformamidine)-1,2,5-oxadiazole- Synthesis of 3-yl)amino)ethyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-pyrrole-2-carboxamide (I-3)
[0068] Synthesis of (Z)-4-amino-N-(3-chlorobenzyl)-N'-hydroxy-1,2,5-oxadiazole-3-carboxamidine (IV-2)
[0069] With compound II-1 (6.9 g, 42.5 mol), 3-chlorobenzylamine (III-2, 5.0 g, 35.4 mmol) and Na 2 CO 3 (5.6g, 53.1mmol) was used as raw material, and the operation was the same as IV-1 to obtain 4.8g of off-white solid IV-2, yield 50.7%, m.p.106.0-108.0℃. 1 H-NMR (400MHz, DMSO-d 6 ), δ(ppm): 10.84(s, 1H, ), 7.31(t, J=7.7Hz, 1H), 7.29–7.23(m, 2H), 7.15(d, J=7.6Hz, 1H), 7.04( t, J=7.2Hz, 1H), 6.29(s, 2H), 4.62(d, J=7.2Hz, 2H).
[0070] 3-(4-Amino-1,2,5-oxadiazol-3-yl)-4-(3-chlorobenzyl)-1,2,4-oxadiazol-5(4H)-one (V -2) Synthesis
[0071] Using compound IV-2 (4.5 g, 16.8 mmol) and CDI (4.0 g, 25.2 mol) as raw materials, and operating the same as V-1, 4.0 g of white solid V-2 was obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com