Therapeutic or prophylactic agent for cachexia
A technology of therapeutic agent and preventive agent, applied in the field of cachexia therapeutic agent or preventive agent, which can solve the problems of ghrelin responsiveness and ghrelin resistance, etc., and achieve the effect of alleviating side effects, improving cachexia symptoms, and improving weight loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
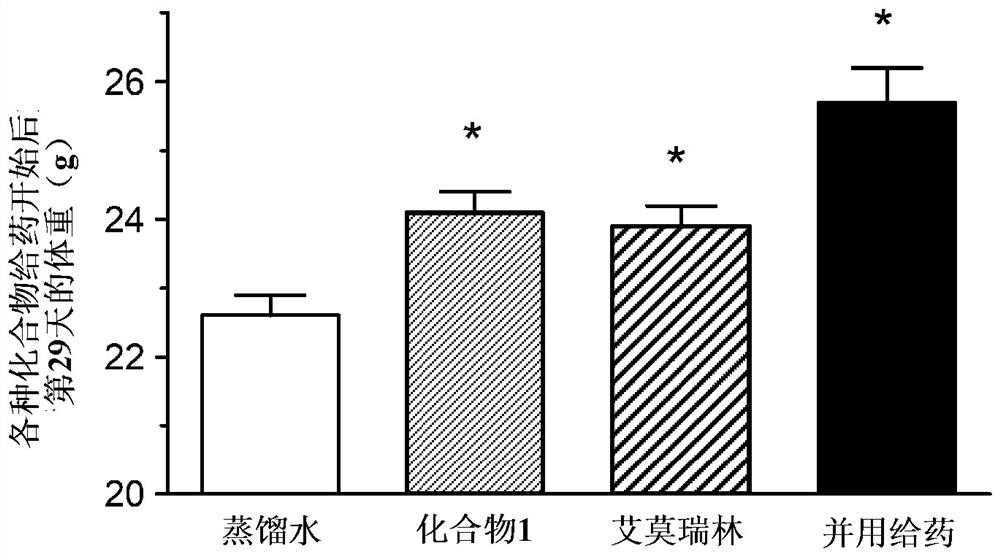
[0119] (Example 1) (-)-17-(cyclopropylmethyl)-3,14β-dihydroxy-4,5α in a mouse cancer-bearing model (a ghrelin-resistant non-small cell lung cancer model) -Effect of combined use of epoxy-6β-[N-methyl-trans-3-(3-furyl)acrylamide]morphinane hydrochloride (below, compound 1) and emmorelin hydrochloride:
[0120] Using A549 cells, which are human alveolar basal epithelial adenocarcinoma cells, transplanted into nude mice, the effect on food intake and body weight when compound 1 was combined with emerin hydrochloride (MedChemExpress) was studied. the medicinal effect.
[0121] Passaging of A549 cells was performed using RPMI1640 medium containing 10% FBS. For the efficacy evaluation, 7-week-old female BALB / C slc / nu / nu mice (SLC from Japan) were purchased and used after one week of acclimation. The preparation of cancer-bearing model animals was carried out as follows. That is, A549 cells were subcutaneously transplanted into the right abdomen of mice, 2.5 × 10 per mouse. 7 ind...
Embodiment 2)
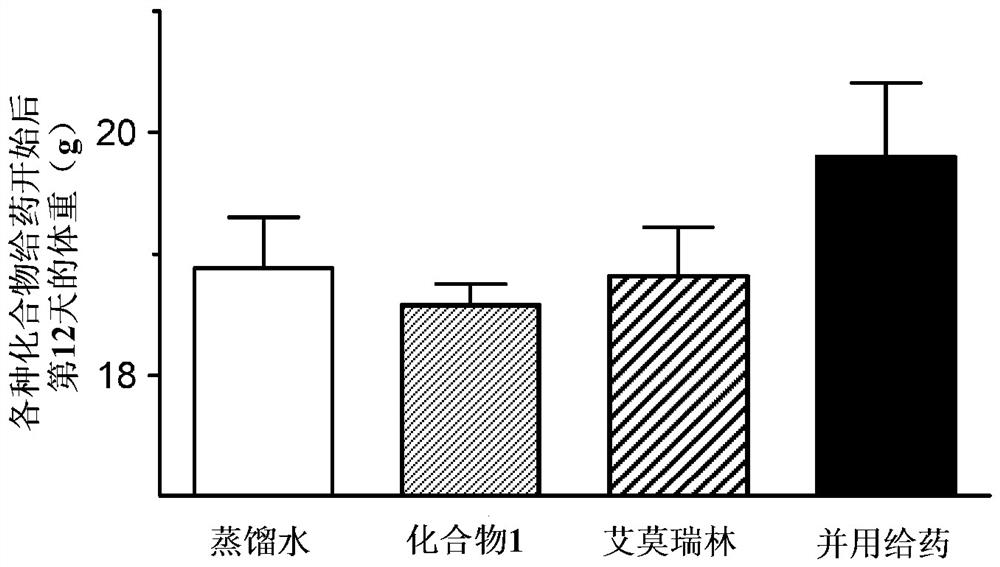
[0126] (Example 2) Combination effect of compound 1 and emmorelin hydrochloride in a mouse cancer-carrying model (non-small cell lung cancer model):
[0127] Using a cancer-bearing model animal obtained by transplanting A549 cells, which are human alveolar basal epithelial adenocarcinoma cells, into nude mice, the pharmacological effects on food intake and body weight when Compound 1 was combined with emmorelin hydrochloride were studied.
[0128] Passaging of A549 cells was performed using RPMI1640 medium containing 10% FBS. For the efficacy evaluation, 7-week-old female BALB / C slc / nu / nu mice (SLC from Japan) were purchased and used after one week of acclimation. The preparation of cancer-bearing model animals was carried out as follows. That is, A549 cells were subcutaneously transplanted into the right abdomen of mice, 2.5 × 10 per mouse. 7 indivual. On the 22nd day after cell transplantation, grouping was performed in such a way that the mean tumor volume of each group ...
Embodiment 3)
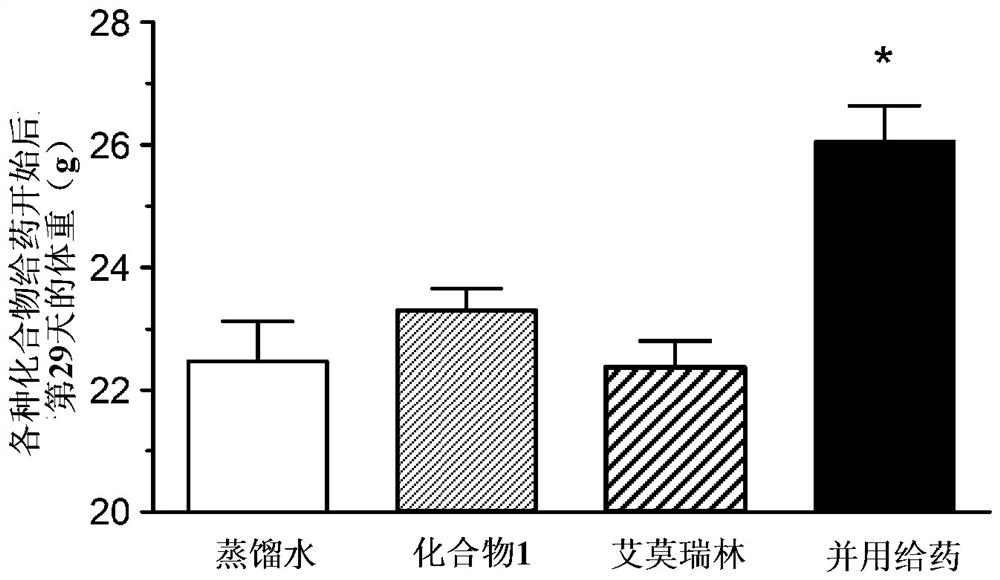
[0132] (Example 3) Combination effect of compound 1 and emmorelin hydrochloride in a mouse cancer-carrying model (skin cancer model):
[0133] Using a cancer-bearing model animal obtained by transplanting B16 / F10 cells, which are mouse malignant melanoma cells, into mice, the pharmacological effects on food intake and body weight when compound 1 was combined with emerin hydrochloride were studied .
[0134] Passaging of B16 / F10 cells was performed using DMEM medium containing 10% FBS and penicillin-streptomycin. For the efficacy evaluation, female C57BL / 6J mice (Charles River, Japan) at the age of 6 weeks at the time of introduction were used, and they were used after acclimation for one week. The preparation of cancer-bearing model animals was carried out as follows. That is, B16 / F10 cells were subcutaneously transplanted into the right abdomen of mice, 5 × 10 per mouse. 6 indivual. On the 5th day after cell transplantation, the groups were divided in such a way that the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com