Prophylactic and/or therapeutic agent for inflammatory lung disease
An inflammatory and therapeutic agent technology, applied in the field of preventive and/or therapeutic agents for inflammatory lung diseases, can solve the problems that cannot be said to be fundamental treatment, cannot expect fibrosis inhibition, improvement, etc., to achieve inhibition of proliferation, inhibition of differentiation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0114] (Reference Example 1) Preparation of S100A8 / A9 Heterodimer for Anti-S100A8 / A9 Antibody Production
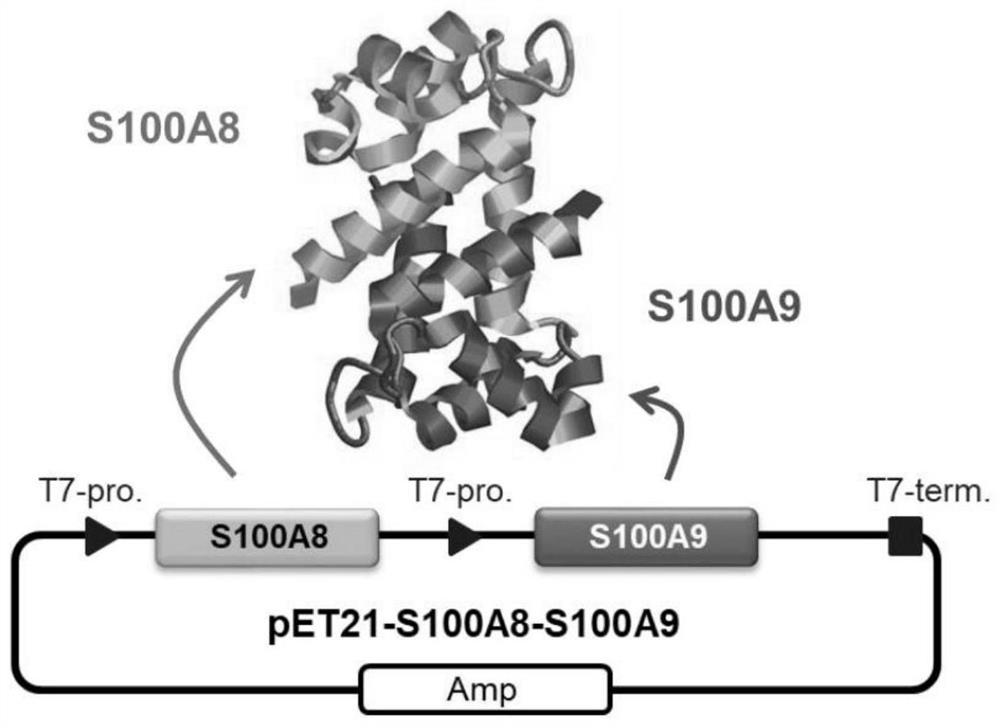
[0115] In this reference example, the preparation of the S100A8 / A9 heterodimer, which is an antigen used for the preparation of the anti-S100A8 / A9 antibody shown in the following examples, will be described. An expression vector obtained by incorporating full-length S100A8 and full-length S100A9 into pET21 (see figure 1 ) S100A8 / A9 heterodimer was produced by E. coli and purified (see Futami J. et al., Biochem Biophys Rep., 19; 6: 94-100, (2016)). As a comparative example, full-length S100A8 or full-length S100A9 was incorporated into pET21, produced in E. coli by the same method as above, and purified (see Futami J. et al. (2016)).
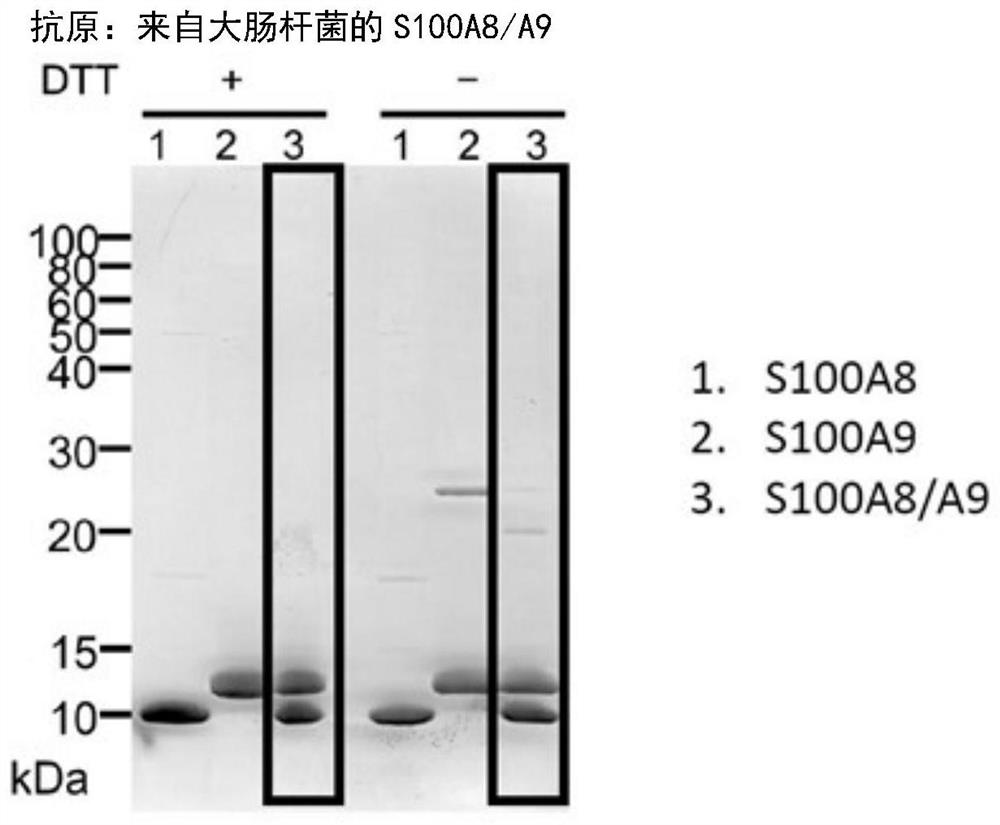
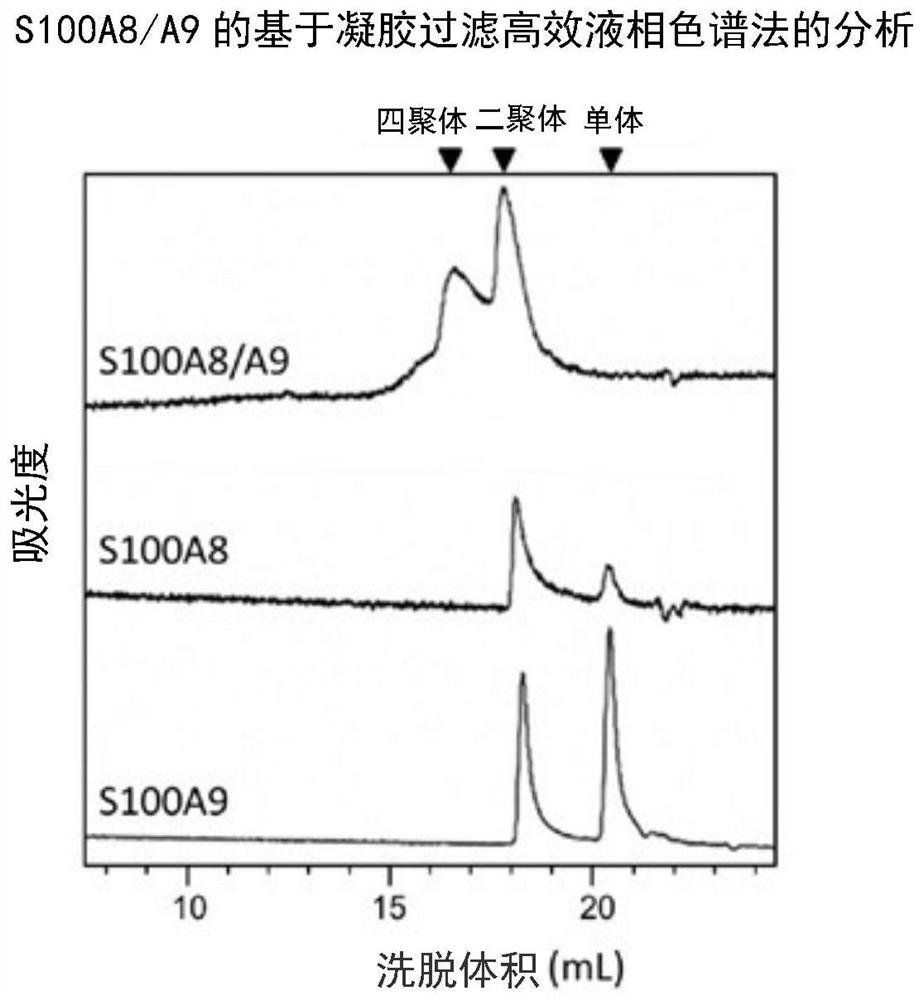
[0116] The purified S100A8 / A9 heterodimer and the S100A8 monomer and S100A9 monomer as comparative examples were subjected to SDS-PAGE and CBB staining, and the results are shown in figure 2 . For the S100A8 / A9 heterodimer, it was confir...
Embodiment 1
[0118] (Example 1) Production of anti-S100A8 / A9 monoclonal antibody
[0119] In this example, the preparation of the anti-S100A8 / A9 monoclonal antibody used in the following examples and experimental examples will be described. The anti-S100A8 / A9 monoclonal antibody of this example was prepared using the S100A8 / A9 heterodimer prepared in the above (Reference Example 1) as an antigen.
[0120] (1) Preparation of hybridomas
[0121] The anti-S100A8 / A9 monoclonal antibody of this example was produced using the S100A8 / A9 heterodimer prepared in the above (Reference Example 1) as an antigen, using a monoclonal antibody customization service, Genostaff (Genetics, Japan). Mice (Balb / c) were used as immunized animals, and Titer-MAX was used as an adjuvant in the immunization of the antigen. According to the conventional method, the spleen of the immunized animal was fused with mouse myeloma cells (P3U1) using polyethylene glycol (PEG1500) to prepare hybridomas, and 160 clones were o...
Embodiment 2
[0128] (Example 2) Screening of neutralizing antibodies
[0129] In this Example, the monoclonal antibodies produced by the 160 cloned hybridomas produced and selected in Example 1 were examined for their influence on the production of S100A8 / A9-induced inflammatory cytokines. Using human keratinocytes in which inflammatory cytokines are strongly induced by S100A8 / A9, the inhibitory effect of each antibody on S100A8 / A9 signaling was evaluated using the mRNA expression levels of inflammatory cytokines as an index. Specifically, 30 ng / mL of purified S100A8 / A9 and each anti-S100A8 / A9 monoclonal antibody obtained by purification from 1 mL of 160 cloned hybridoma culture supernatants by a Protein G column were added to keratinocytes. (Normal Human Keratinocytes: NHK), the cells cultured at 37°C for 3 hours were collected, and the mRNA expression levels of TNF-α, IL-6, and IL-8 were analyzed by real-time quantitative PCR (qPCR).
[0130] Real-time quantitative PCR (qPCR) analysis w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com