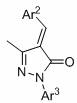
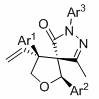
Synthesis method of chiral spiro tetrahydrofuran-pyrazolone compound
A furan pyrazolone and a synthesis method technology are applied in the field of catalytic synthesis of a chiral spirocyclic tetrahydrofuran pyrazolone compound to achieve the effects of improving reaction yield, excellent enantioselectivity and chemical selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
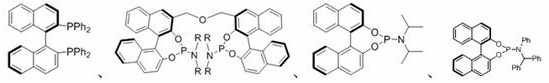
[0041] The reaction flask was sequentially added tetrakistriphenylphosphine palladium (5.7 mg, 0.005 mmol), chiral bisphosphoramidite (7.6 mg, 0.01 mmol), 2 mL of m-xylene was added, stirred at room temperature for 1 hour, and then added 1a (28.5 mg, 0.15 mmol), 2a (26.2 mg, 0.1 mmol), reacted at 10 °C for 24 hours. The target product 3a (yield: 35.9 mg, 88%) can be obtained by simple column chromatography (eluent: ethyl acetate: petroleum ether=100:1) in the reaction system as a brown solid with 99% enantioselectivity , the diastereoselectivity is >20:1.
[0042] The product 3a was analyzed and the results were as follows: 1 H NMR (400 MHz, CDCl 3 ) δ 7.87 (d, J = 8.4Hz, 1H), 7.45 (t, J = 7.6 Hz, 3H), 7.28 (m, 8H), 6.92 (d, J = 8.0 Hz, 1H), 6.78 (dd, J = 17.2, 10.8 Hz, 1H), 5.82 (s, 1H), 5.37 (d, J = 10.4 Hz, 1H), 5.01 (d, J = 8.8 Hz, 1H), 4.92 (d, J = 4.4 Hz, 1H), 4.89 (d, J = 4.0 Hz, 1H),1.63 (s, 3H) ; 13 C NMR (101 MHz, CDCl 3 ) δ 170.6, ...
Embodiment 2
[0050]
[0051] The reaction flask was sequentially added tetrakistriphenylphosphine palladium (5.7 mg, 0.005 mmol), chiral bisphosphoramidite (7.6 mg, 0.01 mmol), 2 mL of m-xylene was added, stirred at room temperature for 1 hour, and then added 1b (30.6 mg, 0.15 mmol), 2a (26.2 mg, 0.1 mmol), reacted at 10 °C for 24 hours. The target product 3b was obtained by simple column chromatography (eluent: ethyl acetate: petroleum ether=100:1) in the reaction system (the yields were 33.7 mg, 80%, respectively) as a white solid with an enantioselectivity of 94 %, diastereoselectivity >20:1.
[0052] The product 3b was analyzed and the results were as follows: 1 H NMR (400 MHz, CDCl 3 ) δ 7.88 (d, J = 8.0Hz, 1H), 7.46 (t, J = 8.0 Hz, 2H), 7.34–7.23 (m, 6H), 7.10 (d, J = 7.6 Hz, 2H), 6.81 (d, J = 7.6 Hz, 2H), 6.76 (dd, J = 17.2, 10.8 Hz, 1H ), 5.82 (s, 1H), 5.36 (d, J = 10.6 Hz, 1H), 5.00 (d, J = 8.8 Hz, 1H), 4.91 (m, 2H), 2.31(s, 3H), 1.65 (s, 3H) ; 13 C NMR (101...
Embodiment 3
[0054]
[0055] The reaction flask was sequentially added tetrakistriphenylphosphine palladium (5.7 mg, 0.005 mmol), chiral bisphosphoramidite (7.6 mg, 0.01 mmol), 2 mL of m-xylene was added, stirred at room temperature for 1 hour, and then added 1d (40.2 mg, 0.15 mmol), 2a (26.2 mg, 0.1 mmol), reacted at 10 °C for 24 hours. The target product 3d (yield 40.8 mg, 84%, respectively) can be obtained by simple column chromatography (eluent: ethyl acetate: petroleum ether=100:1) in the reaction system as a white solid with an enantioselectivity of 95 %, diastereoselectivity >20:1.
[0056] The product 3d was analyzed and the results were as follows: 1H NMR (400 MHz, CDCl3) δ 7.81 (d, J = 8.0Hz, 2H), 7.44 – 7.37 (m, 4H), 7.23 (m, 6H), 6.76 (d, J = 8.8 Hz, 2H), 6.72(dd, J = 17.6, 10.8 Hz, 1H), 5.78 (s, 1H), 5.34 (d, J = 10.4 Hz, 1H), 4.92(d, J = 8.8 Hz, 1H), 4.88–4.79 (m, 2H), 1.60 (s, 3H); 13C NMR (101 MHz, CDCl3) δ IR (KB)ν; max : 3015, 2923, 1703, 1595, 1498, 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com