Desorption composition of adsorbed vaccine containing cpg ODN and its application
A desorption and adsorption type technology, applied in the field of determination of the efficacy of adjuvanted vaccines, can solve the problems of no reported CpGODN composite adjuvant adsorption adsorption type vaccine desorption method, time-consuming, vaccine inapplicability, etc. The effect of immune activity, easy promotion and application, and rapid separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] This implementation provides a method for detecting antigen efficacy in a CpG ODN composite adjuvant adsorption vaccine, the specific steps are as follows:
[0064] Step 1: Preparation of desorption reagents: wherein the final molar concentration of potassium phosphate is 0.6M, the final molar concentration of magnesium chloride is 0.1M, the final volume concentration of Triton-100 is 1%, and the final volume concentration of M199 medium is 20% ;
[0065] Step 2: Desorb the CpG ODN composite adjuvant-adsorbed vaccine reference substance and the sample to be tested: Mix the reference substance and sample with the desorption reagent in a volume ratio of 1:1, incubate at 28°C for 60 minutes, and incubate every 20 minutes during the incubation process. Invert and mix once;
[0066] The obtained mixture was centrifuged, the centrifugation program was 6000rpm, the centrifugation time was 20min, and the centrifugation temperature was 4°C to obtain a supernatant;
[0067] Ste...
Embodiment 2
[0075] This implementation provides the results of antigen dissociation in different batches of CpG ODN composite adjuvant-adsorbed novel coronavirus inactivated vaccines, and examines the applicability of the dissociation method to different batches of samples.
[0076] The specific desorption and antigen potency determination methods are the same as those in Example 1.
[0077] Table 4 shows the test results of antigen potency in different batches of CpG ODN composite adjuvant-adsorbed novel coronavirus inactivated vaccines.
[0078] Table 4
[0079]
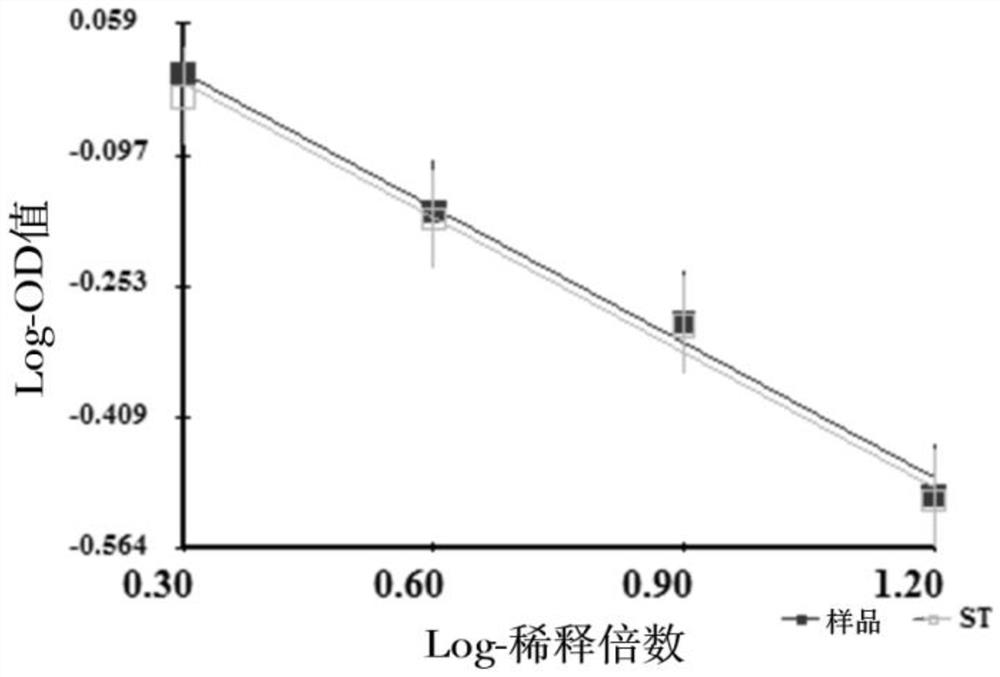
[0080] Sample 1-5 double parallel lines result as figure 2 shown.
[0081] As can be seen from Table 4, the present invention has good applicability to the CpG ODN composite adjuvant adsorption type novel coronavirus inactivated vaccine.
Embodiment 3
[0083] This example verifies the applicability of desorption of CpG ODN composite adjuvant-adsorbed new inactivated vaccines using desorption compositions with different component ratios, different desorption conditions, and different centrifugation parameters.
[0084] The theoretical antigen concentration of the sample to be detected used in this example is 13U / ml, and the sample dilution scheme is 2X, 4X, 8X, and 16X. Antigen potency test results between 0.5-2.0 are considered qualified.
[0085] Table 5 shows the desorption conditions used in each sample group and the detection results of antigen potency.
[0086] The specific antigen potency determination operation procedure is the same as that in Example 1.
[0087] table 5
[0088]
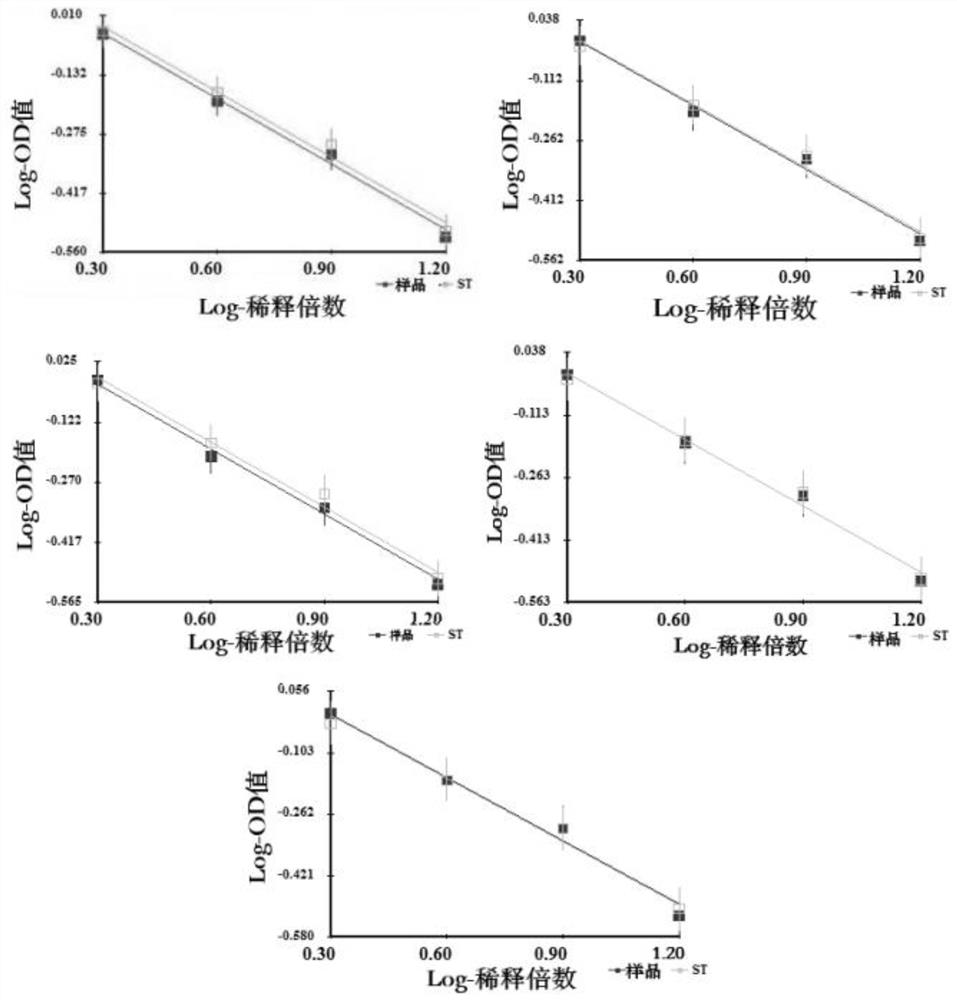
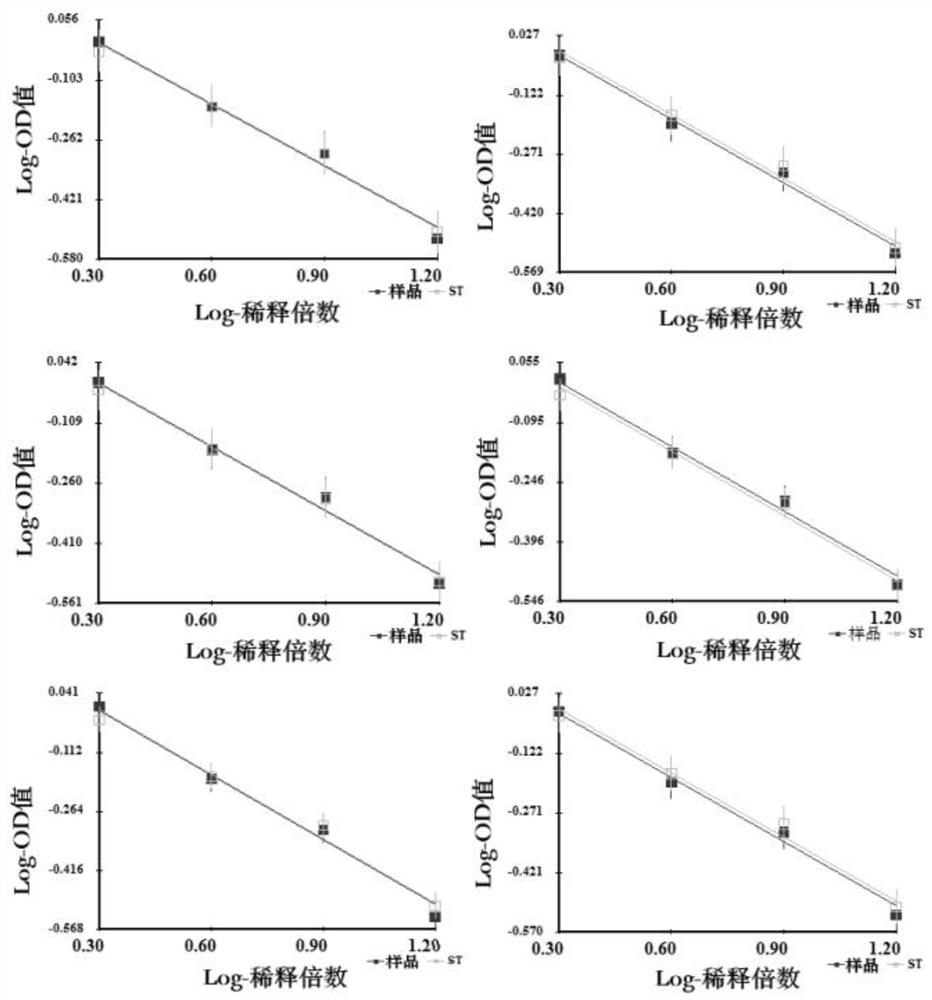
[0089] Samples 1-6 double parallel line results such as image 3 shown.
[0090] It can be seen from Table 5 that the test results are all qualified, and it is verified that the desorption composition, the desorption reagent and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com